Anxiety in Huntington’s Disease
Abstract
Anxiety is common in Huntington’s disease (HD), though it has been under-researched. The authors conducted a systematic review of anxiety in HD. The prevalence of anxiety in manifest HD ranged from 13% to 71%. No significant difference in anxiety between manifest and premanifest HD carriers was revealed. Anxiety appears to be associated with depression, suicide, irritability, quality of life (QoL), pain, illness beliefs, and coping styles but does not seem to be linked with measures of disease progression. From the few pilot studies available, interventions that show promise include olanzapine and psychosocial approaches. Improved assessment, more exploration of the nature of anxiety in HD, and evaluation of anxiety interventions are required.
Huntington’s disease (HD) is a genetic neurodegenerative disease characterized by motor disturbance, cognitive decline, and psychiatric symptoms. HD is caused by an expansion of cytosine-adenine-guanine (CAG) repeats in the Htt gene on the short arm of chromosome 4, resulting in production of the mutant protein huntingtin. The exact pathophysiological processes that occur in HD are not yet fully understood, although different mechanisms have been suggested.1 The onset of the disease usually occurs in midadulthood, with considerable variation in clinical presentation. The disease is autosomal dominant, which means that those who carry the gene present a 50% risk of passing the disease to any of their children. The duration of the disease varies but is, on average, 20 years from the onset of motor signs to death.2 As yet, no cure has been identified for HD, although treatments to manage different symptoms may help reduce distress and improve QoL.
So far, the clinical diagnosis of HD is dependent on the motor signs of the disease, involving problems with voluntary and involuntary movements. However, cognitive and psychiatric symptoms may be present many years before the onset of motor problems.3–5 The psychiatric component of the disease can involve a number of different complaints including depression, irritability, anxiety, apathy, obsessive-compulsive behaviors (OCBs), and psychosis.6,7 Although motor and cognitive symptoms worsen with time, psychiatric symptoms appear to have a more variable course,8 with the exception of apathy, which is more prevalent in advanced disease stages.
A systematic review of psychopathology in HD identified that the prevalence of anxiety among manifest HD participants was between 34% and 61%, depending on the disease stage and the measure used.7 Although anxiety symptoms are commonly reported in HD, this topic has received limited attention in the literature, with the exception of OCBs, which have been reported on more frequently.9 However, it is questionable whether OCBs in HD may be construed as an anxiety condition, as there is evidence that they may be more closely associated with the cognitive changes in HD, particularly executive dysfunction.10
Because there is a lack of information about anxiety in HD, we systematically reviewed the literature on the epidemiology and phenomenology of anxiety in HD and identified areas for future study.
Methods
We carried out data searches using PsycINFO and MEDLINE to identify published articles from 1994–2014. The rationale for our exclusion of articles written before 1994 was due to predictive genetic testing only becoming available after the discovery of the HD gene in 1993. Searches were restricted to human studies that were written in English. The following search strings were used in February 2014: “Huntington*” combined with “anxiety*,” “psychiatric,” and “psychological.” The term “depression” was also combined with “Huntington*,” as we were aware that some studies of anxiety in HD are embedded within broader research of depression in HD and that some studies using measures of psychopathology, developed specifically for use within an HD population, combined factors for anxiety and depression.
From the database searches, we retrieved a total of 209 articles from MEDLINE and 230 from PsycINFO. An additional eight articles were identified from reference lists and consultation with researchers in the field. Duplicates were removed, resulting in 297 articles. We then conducted a manual search of titles and abstracts and excluded the following: case studies, conference abstracts, qualitative studies or studies with no standardized measure of anxiety, reviews, dissertation abstracts, juvenile HD studies, and studies not including verified HD expansion carriers (e.g., involving only at-risk or caregivers of HD). We also excluded articles that were confined only to obsessive-compulsive disorder (OCD) because it could be argued that these symptoms are more closely related to the cognitive symptoms of HD, rather than anxiety.
Following this, a total of 70 studies warranted manual full-text searches to assess eligibility. Reasons for article exclusion at this stage were that they did not include a standardized measure of anxiety (N=20), they were review articles (N=4), or the sample included participants with disorders other than HD (N=1). The total number of studies finally included was 45. See Figure 1 for a summary of the review process.
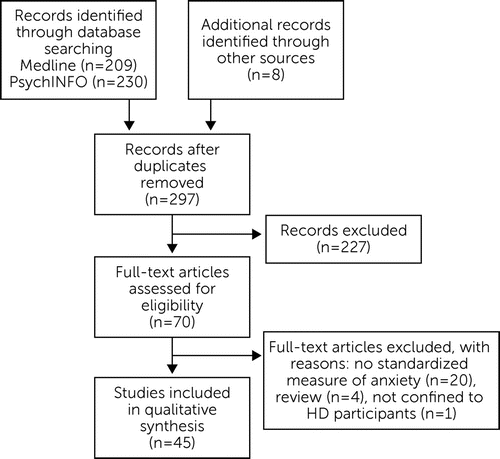
FIGURE 1. Flowchart Illustrating the Number of Articles Excluded at Each Stage of Eligibility Screening
Results
Using the eligibility criteria, we found the following studies: 16 articles reporting on prevalence studies (Table 1), 13 articles describing group comparisons (Table 2), 28 articles reporting correlates and predictors (Table 4), and five articles describing interventions (Table 5). Sixteen studies fell into more than one group. None of the studies examined anxiety as the primary theme of the study.
| Study | No. of Participants | Measure | Disease Duration (years), Mean (SD) [range] | Disease Stage | Country | Prevalence |
|---|---|---|---|---|---|---|
| Manifest HD | ||||||
| Murgod et al. (2001)12 | 26 | UHDRS | 5.5 (3.9) [1–20] | 1–5 | India | 61.5% (point prevalence) |
| Paulsen et al. (2005)8 | 2,835 | UHDRS | 7.6 (6.0) | 1–5 | U.S. | 41% (point prevalence) |
| Craufurd et al. (2001)6 | 134 | PBA-HD | 9±5 [1–23] | 1–5 | UK | 37% (point prevalence) |
| Thompson et al. (2012)15 | 111 | PBA-HD | 5.5 (4.7) | 1–5 at baseline | UK | 71% (cumulative prevalence at 3 years) |
| Kulisevsky et al. (2001)17 | 29 | NPI | 5.6±1.6 | Not given | Spain/U.S. | 34% (point prevalence) |
| Paulsen et al. (2001)18 | 52 | NPI | 4.7 (4.4) [1–20] | Not given | U.S. | 51.9% (point prevalence) |
| Kristjanson et al. (2006)20 | 46 | HADS | 7.8 (4.8) | Not given | Australia | 41% (point prevalence) |
| Arran et al. (2014)21 | 165 | HADS | Not given | Not given | UK | 48% point prevalence |
| Leroi et al. (2002)26 | 21 | SCID | 12 (6.6) | Not given | U.S. | 24% (lifetime prevalence) |
| Vassos et al. (2008)24 | 72 | SCID | 5.62 (5.7) | Not given | Greece | 12.5%, (point prevalence) |
| 16.7% (lifetime prevalence) | ||||||
| van Duijn et al. (2008)25 | 85 | CIDI | Not given | Not given | Netherlands | 16.5% (12-month prevalence) |
| Premanifest HD | ||||||
| Berrios et al. (2001)28,a | 26 | CIDI | N/A | N/A | UK | 11.5% (point prevalence) |
| STAI | ||||||
| Berrios et al. (2002)29,a | 32 | CIDI | N/A | N/A | UK | 0% (point prevalence) |
| STAI | ||||||
| Shiwach and Norbury (1994) 31,a | 20 | PSE | N/A | N/A | UK | 0% (point prevalence) |
| Julien et al. (2007)30,a | 89 | CIDI | N/A | N/A | UK | 15% (point prevalence) |
| 17% (lifetime) | ||||||
| van Duijn et al. (2008)25,b | 55 | CIDI | N/A | Not given | Netherlands | 14.5% (12-month prevalence) |
| All HD mutation carriers | ||||||
| Reedeker et al. (2012)27 | 142 at baseline, | CIDI | Not given | 1–2 | Netherlands | 17% (2-year cumulative prevalence) |
| 106 at follow-up |
TABLE 1. Prevalence of Anxiety in HD Expansion Carriers
| Group Comparisons | Noncarriers (N) | Premanifest HD (N) | Manifest HD (N) | Measure of Anxiety Used | Outcome |
|---|---|---|---|---|---|
| Manifest versus premanifest | |||||
| Soliveri et al. (2002)23,s | — | 17 | 40 | HAM-A | Manifest>premanifest |
| Marshall (2007)4,b | — | 49 | 34 | SCL-90 | NS |
| van Duijn et al. (2008)25,a | — | 55 | 85 | CIDI | NS |
| Manifest versus noncarrier,c | |||||
| Soliveri et al. (2002)23,a | 28 | — | 40 | HAM-A | Manifest>noncarrier |
| van Duijn et al. (2008)25,a | 56 | — | 85 | CIDI | NS |
| Premanifest versus noncarrier | |||||
| Shiwach and Norbury (1994)31,d | 33 | 20 | — | PSE | NS |
| Rosenberg et al. (1995)55,d | 19 | 14 | — | SCL-90 R | NS |
| Decruyenaere et al. (1995)56,d | Male=12 | Male=13 | — | STAI | Male carriers significantly less anxious than noncarriers, no difference for females. No analysis for total sample of males and females. |
| Female=23 | Female=12 | ||||
| Decruyenaere et al. (1996)22,c | 31 | 22 | — | STAI | NS at pretest and post-test |
| Decruyenaere et al. (1999)44,c | 40 | 29 | — | STAI | NS at pretest and 1 year posttest |
| Soliveri et al. (2002)23,a | 28 | 17 | — | HAM-A | NS |
| Berrios et al. (2002)29,d | 66 | 32 | — | CIDI | NS |
| Witjes-Ane et al. (2002)14,c | 88 at baseline, | 45 | — | UHDRS | NS at pretest and 18 months posttest |
| 78 at 18 months | 35 | ||||
| Decruyenaere et al. (2003)45,c | 33 | 24 | — | STAI | NS pretest and 5 years posttest |
| Duff et al. (2007)3,a | 92 | 589 | — | SCL-90 R | Premanifest>noncarrier |
| Marshall et al. (2007)4,b | 171 | 49 | — | SCL-90 R | Premanifest>noncarrier |
| Julien et al. (2007)30,d | 115 | 89 | — | CIDI | NS |
| van Duijn et al. (2008)25,a | 56 | 55 | — | CIDI | NS |
TABLE 2. Group Comparisons of Anxiety in HD Expansion Carriers and Noncarriers
| Study | Participants (N) | Agoraphobia (%) | GAD (%) | Panic Disorder (%) | Simple Phobia (%) | Social Phobia (%) |
|---|---|---|---|---|---|---|
| Berrios et al. (2001)28,a | Pre-HD (26) | — | 3.8 | 7.7 | — | — |
| Julien et al. (2007)30,a | Pre-HD (89) | 6 | 9 | 6 | 7 | 2 |
| van Duijn et al. (2008)25,b | Pre-HD (55) | 1.8 | 5.5 | 3.6 | — | 5.5 |
| HD (85) | 1.2 | 4.7 | 4.7 | 5.9 | ||
| Reedeker et al. (2010)27 | All HD expansion carriers (142 at baseline, 106 at follow-up) | 2.8 | 5.7 | 5.7 | — | 7.5 |
TABLE 3. Prevalence of Individual Anxiety Disorders Among HD Expansion Carriers
| Study | No. of Participants | Measure of Anxiety | Outcome |
|---|---|---|---|
| Jankovic et al. (1995)46 | 36 HD | UHDRS | Assessed impact of genetic testing and found no change in anxiety before and after testing |
| Decruyenaere et al. (1996)22 | 22 pre-HD | STAI | No change in anxiety before and 1 year after genetic testing |
| Levy et al. (1998)37 | 34 HD | NPI | Apathy did not correlate with anxiety. A relationship between anxiety and depression was found |
| Decruyenaere et al. (1999)44 | 29 HD | STAI | No change in anxiety before and 1 year after genetic testing |
| Craufurd et al. (2001)6 | 134 HD | PBA-HD | Anxiety loaded on to depression, but not irritability or apathy. No relationship was found between anxiety and duration of illness |
| Paulsen et al. (2001)18 | 52 HD | NPI | Gender, age, apathy, illness duration, chorea severity, dementia severity, euphoria, disinhibition, and delusions did not correlate with anxiety. Agitation, irritability, and dysphoria did correlate with anxiety |
| Witjes-Ane et al. (2002)14 | 35 pre-HD | UHDRS | Gender and age were not associated with anxiety. No change in anxiety 18 months after testing |
| Decruyenaere et al. (2003)45 | 24 pre-HD | STAI | There was a significant reduction in trait anxiety 5 years after predictive testing and state anxiety 1 year and 5 years after testing |
| Marshall et al. (2007)4 | 49 pre-HD | SCL-90 R | Premanifest HD carriers had more motor abnormalities. Although not reaching manifest HD, they had more anxiety than premotor symptomatic carriers with no or minor nonspecific motor abnormalities, but this finding did not reach significance |
| Paulsen et al. (2005)8 | 2,835 HD | UHDRS | A higher level of anxiety was found in stage 2 disease than in other stages; after stage 2, a progressive decrease in anxiety was reported |
| Julien et al. (2007)30 | 89 pre-HD | CIDI | Presence of anxiety disorders was not related to proximity to onset of manifest HD |
| Kingma et al. (2008)38 | 152 all carriers | PBA-HD | Anxiety loaded onto depression factor, not irritability or apathy |
| Zappacosta et al. (2008)32 | 29 HD | HAM-A | Anxiety correlated with depression but not with CAG repeats, motor ability, cognitive functioning, and duration of illness |
| Leroi et al. (2002)26 | 21 HD | SCID | Anxiety was not associated with gender |
| Vassos et al. (2008)24 | 72 HD | SCID | CAG repeats were not associated with anxiety |
| McCabe et al. (2009)34 | 48 HD | POMS-SF | Anxiety-tension made a significant contribution to QoL |
| Marder et al. (2010)35 | 960 HD | UHDRS | Anxiety loaded onto depression factor. Severity on the depression/anxiety factor of the UHDRS influenced the rate of decline on the Independence Scale |
| Litvan et al. (1998)33 | 29 HD | NPI | Positive relationship between agitation/irritability and anxiety No relationship was found between the degree of chorea and anxiety |
| Thompson et al. (2012)15 | 86 HD | PBA-HD | Did not find an increase in anxiety across stages of disease progression |
| Wetzel et al. (2011)41 | 1941 HD | UHDRS | Depression/anxiety was found to significantly predict suicidal ideation and the top most 25% most severe suicidal ideation among the sample |
| Soliveri et al. (2002)23 | 40 HD | HAM-A | Anxiety was associated with depression but not with CAG repeat length, motor ability, cognitive functioning, or disease duration |
| Rickards et al. (2011)39 | 1803 HD | UHDRS | Support for a cluster of symptoms involving anxiety and depression was found in this factor analysis study |
| Rickards et al. (2011)40 | 768 HD | HAM-D | Anxiety item on the HAM-D was not good at discriminating between HD patients who were or were not experiencing depressed mood |
| Nimmagadda et al. (2011)36 | 30 HD | IDA, STAI | State and trait anxiety were associated with inward and outward irritability; state and trait anxiety correlated with depression |
| Berrios et al. (2001)28 | 26 pre-HD | CIDI, STAI | No relationship between anxiety and CAG repeat length |
| Hubers et al. (2013)43 | 2106 HD | UHDRS | Anxiety was independently associated with suicidal ideation at baseline, but during a 4-year period it was not predictive of suicidal ideation |
| Fiedorowicz et al. (2011)42 | 735 pre-HD | PSS | Anxiety was not found to be an independent risk factor for suicidal behavior, defined as suicide or attempted suicide, across a mean of 3.7 years’ prospective follow-up |
| Arran et al. (2014)21 | 165 HD | HADS | Anxiety was associated with pain, high number of symptoms, less strong belief in treatment control, and stronger belief in fluctuation of illness. Coping strategies associated with high anxiety were low acceptance, venting, self-blame, and behavioral disengagement |
TABLE 4. Studies of Correlates and Predictors
| Study | No. of Participants | Intervention | Methodology | Measure of Anxiety Used | Outcome |
|---|---|---|---|---|---|
| Murman et al. (1997)57 | 10 | Ketamine at 0.10, 0.40, and 0.60 mg/kg/hr | Double-blind, placebo-controlled, 1-day testing of increasing doses | BPRS and SRS | Nonsignificant |
| Squitieri et al. (2001)48 | 10 | Olanzapine at 5 mg dose orally once per day | Open pilot, no control group, no blinding; 6-month intervention | UHDRS | Significant improvement at p=0.016 |
| Lundin et al. (2010)58 | 28 treatment, 30 control, All manifest HD | Pridopidine at 50 mg/day | Randomized, double-blind, placebo-controlled 4-week trial | HADS | Nonsignificant trend for improvement |
| A’Campo et al. (2012)47 | 29 HD with caregivers 12 pre-HD with partners | PEP-HD | Pilot study, no control group | HADS | Significant improvement at p=0.05 for manifest HD Nonsignificant for premanifest HD |
| Piira et al. (2013)49 | 37 | Intensive multidisciplinary program | Pilot study, no control group | HADS | Significant improvement at p=0.001 |
TABLE 5. Interventional Studies
Prevalence and Group Comparison Studies
Prevalence studies are presented in Table 1. The prevalence of anxiety ranged from 13% to 71% in manifest HD participants and from 0% to 15% in premanifest carriers. Of these studies, 11 examined prevalence in manifest HD participants, five involved premanifest participants, and one study involved both manifest and premanifest carriers. Studies that compared the presence of anxiety in HD expansion carriers and verified noncarriers are presented in Table 2.
HD-specific measures.
HD-specific assessments used to measure prevalence were the United Huntington’s Disease Rating Scale (UHDRS)11 and the Problem Behaviors Assessment for Huntington’s Disease (PBA-HD).6 The UHDRS has one item that measures frequency and severity of anxiety on a 5-point scale. The PBA-HD has one item that relates to anxiety, with the longer version also having an item on tension.
Two studies used the UHDRS among manifest HD participants and reported prevalence varying from 41% (N=2,835)8 to 62% (N=26).12 One factor that may account for the differences in these rates is that the former study used a cutoff score of greater than 3 when frequency was multiplied by severity on the UHDRS, whereas the latter study did not indicate a precise cutoff score; rather, the authors stated that there were “behavioural disturbances of some degree.” Also, the first study involved a U.S. sample,8 whereas the second study was conducted in India.12 Both studies included participants from across all five disease stages,13 although the U.S. study included a large majority (85% of the sample) with disease stages 1–3. This large-scale U.S. study was the only article identified that examined anxiety by disease stage, defined by a Total Functioning Capacity score of the UHDRS.13 A higher level of anxiety was found in stage 2 than in other stages of the disease; after stage 2, there was a progressive decrease in anxiety.
The UHDRS was used in only one study that examined HD carriers not yet manifest for the disease.14 Anxiety in a premanifest group was compared with a control group of noncarriers, and no differences in anxiety were found between the groups before predictive genetic testing or 18 months after test results were received. The PBA-HD was used to examine the prevalence of anxiety in HD in two studies. A cross-sectional study using the PBA-HD involving 134 UK HD participants showed a point prevalence of 37%,6 whereas a longitudinal study using the PBA-HD reported a prevalence of 71% in 111 UK HD participants across 3 years.15 In both studies, participants were said to experience anxiety if they scored ≥2 on the severity scale of the PBA-HD. No studies were identified that used this instrument to assess anxiety among premanifest carriers or noncarriers.
General symptomatology measures of anxiety.
Assessments of general symptomatology measures of anxiety were used for four studies involving manifest carriers. Two studies used the Neuropsychiatric Inventory (NPI),16 which has one domain that inquires about anxiety, with severity and frequency of symptoms rated by an informed caregiver. One study using the NPI obtained a prevalence rate of 51.9% (N=52),18 where participants who received a score of ≥1 on the NPI were viewed as endorsing anxiety. The other NPI study stated that 34% of 29 HD participants demonstrated “frequency of changes” in anxiety.17
The Hospital Anxiety and Depression Scale (HADS),19 which has seven questions relating to anxiety symptoms, each scored on a 4-point scale, was used in two studies. In one of these studies, a cutoff value of ≥8 was used to identify anxiety cases, obtaining a prevalence of 48%21; the other study did not provide a figure for a cutoff point, instead stating that 41% of their sample reported moderate to severe anxiety.20
Two studies used general symptomatology measures, the Symptom Checklist (SCL)-90 and Hamilton Anxiety Scale (HAM-A), to compare anxiety in manifest HD with premanifest HD4,23; no difference was found in mean scores between the groups. These two studies also compared anxiety levels among manifest HD patients with noncarriers and found that manifest HD participants reported more anxiety symptomology than noncarriers.
Eight studies examined differences in mean anxiety scores between premanifest HD carriers and noncarriers using general symptomatology measures. The majority of these studies found no significant difference, although two studies with the largest sample sizes found that premanifest HD carriers reported more anxiety3,4 (see Table 2). Three studies examined premanifest carriers and noncarriers longitudinally to assess anxiety pre- and postpredictive testing and found no difference between the premanifest group and noncarriers before or after testing up to 5 years after disclosure of their genetic status.
Diagnostic measures.
Three studies used a structured diagnostic interview, either the Composite International Diagnostic Interview (CIDI) or Structured Clinical Interview for DSM-IV (SCID) to assess the prevalence of formal anxiety disorders in manifest HD carriers. These measures resulted in a lower prevalence compared with the studies based on symptoms with 12.5% (point prevalence), 16.7% (lifetime prevalence) (n=72),24 16.5% (12-month prevalence) (n=85)25, and 24% (lifetime prevalence) (N=21)26 for any anxiety disorder. One longitudinal study using the CIDI investigated a combined sample of premanifest and manifest HD carriers27 and found a 2-year cumulative prevalence of 17% for all expansion carriers.
Five studies using diagnostic measures reported prevalence ranges from 0% to 17% for premanifest carriers.25,28–31 The studies reporting that none of their samples fitted diagnostic criteria29,31 were small studies, whereas another small study (N=26) reported that 11.5% of its sample fitted diagnostic criteria for an anxiety disorder.28 The two studies of premanifest HD expansion carriers with larger sample sizes (N=55 and N=89) reported similar prevalence levels to manifest HD carriers, both using CIDI at 14.5% and 15%.25,30 The lifetime prevalence was estimated as 17% in the latter study.
One of the diagnostic studies compared manifest and premanifest HD carriers using CIDI and found no statistical difference between the groups for formal diagnoses of panic disorder, agoraphobia without panic, generalized anxiety disorder (GAD), social phobia, and OCD.25 The prevalence of formal anxiety disorders in the manifest HD group compared with the noncarrier group was not statistically significant. Four studies using diagnostic criteria found no difference in the frequency of anxiety disorders between premanifest HD carriers and noncarriers.25,29–31
Prevalence of individual anxiety disorders.
The prevalence of formal anxiety disorders is provided in Table 3. The disorders that tended to be more prevalent were GAD (3.8%−9%) and panic disorder (3.6%−7.7%). Prevalence estimates for social phobia were 2%−7.5%, and agoraphobia 0%−6%. Simple phobia was only reported in one study with a prevalence of 7%, and posttraumatic stress disorder was not examined.
Correlates and Predictors
A total of 28 studies were identified that examined the relationship between anxiety and sociodemographic, clinical, psychiatric, and psychological factors in HD.
Sociodemographic and Clinical
Age and gender were not found to relate to anxiety.14,18,26 Four studies that examined the relationship between anxiety and CAG repeat length revealed no association.23,24,28,32 No relationship was found between anxiety and motor functioning. Measures included degree of chorea, aberrant motor behavior18,33 and motor compromise as assessed by Folstein’s Quantified Neurological Examination.23,32 Anxiety was not associated with cognition across three studies, nor was any difference found in anxiety between patients who had dementia (N=11) and did not have dementia (N=18).31 In one study, pain was found to be a significant independent predictor of anxiety.21
Only one study examined whether anxiety symptoms were related to QoL in HD.34 Using regression analysis, for 48 HD participants, these authors found that anxiety-tension response on the Profile of Mood States (short form) (POMS-SF) made a significant contribution to QoL along with physical symptoms, psychological symptoms, and depression.
Only one study was identified that examined anxiety and general daily functioning in HD. This prospective study examined 960 participants35 who were followed up for a mean duration of 18.3 months. These authors found that severity on a combined factor of depression and anxiety on the UHDRS influenced the rate of decline on the Independence scale of the UHDRS.
Other studies have consistently found no relationship between anxiety and disease duration.6,15,18,23,32
Psychiatric
Several correlation studies using a variety of measures found an association between anxiety and depression.18,23,31,36,38 Depression was associated with anxiety in factor analysis studies of the PBA-HD6,39 and in studies using the behavioral section of the UHDRS.35,39 On the other hand, a discriminant analysis of depression measures found that the anxiety item of the HAM-D was not good at discriminating between HD patients who were experiencing depressed mood and those who were not, as measured by a single item on the UHDRS.40
A positive relationship between anxiety and agitation/irritability among manifest HD participants18,33,36 was found. Anxiety appeared to be unrelated to apathy across several studies using correlational methods of the NPI18,37 and factor analysis of the PBA-HD.6,38
Three studies were identified that examined the relationship between anxiety and suicide, with mixed results. In a regression study, a mixed depression/anxiety factor of the UHDRS data for 1,941 motor symptomatic HD mutation carriers was found to significantly predict suicidal ideation.41 However, no separate analysis of depression and anxiety was conducted to examine whether there was any independent association between anxiety and suicidal ideation. No independent risk factor for suicidal behavior, defined as suicide or attempted suicide, was found in premanifest individuals in the PREDICT-HD study.42 Another study using the UHDRS examined 2,106 HD mutation carriers identified anxiety as being independently associated with suicidal ideation at baseline, but during a 4-year period it was not predictive of suicidal ideation.43
Psychological Variables
One study of manifest HD patients identified a number of illness perceptions and coping styles to be associated with higher levels of anxiety.21 A stronger belief in the fluctuation of symptoms, greater illness identity, and a less strong belief in treatment control were related to greater anxiety.21 Coping strategies of venting, self-blame, and behavioral disengagement were associated with higher levels of anxiety; a low acceptance of illness was also linked with higher anxiety.21
There are mixed results regarding the impact of genetic testing on anxiety levels among HD expansion carriers. Studies by the same authors revealed no differences in pre- and posttest trait anxiety in HD expansion carriers 1 year after predictive genetic testing,22,44 but there were significant reductions in trait anxiety 5 years after predictive testing and in state anxiety 1 year and 5 years after testing.45 One study found no change in anxiety among 35 premanifest carriers 18 months after testing using the UHDRS.14 The one study that examined the impact of diagnostic DNA testing on those with motor signs demonstrated no change in anxiety scores at 2-week and 3-month follow-ups.46
Intervention
Only five studies examined potential effects that a medical or psychosocial intervention might have on anxiety in HD (Table 5). Three studies reported a significant improvement in anxiety postintervention.47–49 The first one was an open-label study of olanzapine (5-mg dose orally once per day) during 6 months, involving just 10 participants.48 Another study involved 29 HD expansion carriers and their caregivers who undertook the Participant Education Program for HD (PEP-HD),47 and a third study involved 37 participants who engaged in a range of intensive therapeutic activities during three inpatient stays of 3 weeks across 1 year.49 The PEP-HD study involved HD mutation carriers and their caregivers who undertook 8 two-week sessions of 90 minutes’ duration. Groups were divided into those who were motor symptomatic and premotor symptomatic. The program involved encouraging participants to be proactive in finding information about the disease, self-monitoring, undertaking pleasant activities, performing stress-management techniques, developing coping strategies for depression and anxiety, engaging in social communication, and seeking support. The other intensive therapeutic study involved training activities to improve physical and cognitive functioning, activities of daily living, sessions in the gymnasium and/or swimming pool, and assistive technology assessments. Additionally, patient educational sessions and group discussions were provided. Input was provided from the multidisciplinary team for up to 8 hours per day, 5 days per week.
The olanzapine study48 used the UHDRS to measure anxiety, whereas the other two studies47,49 used the HADS. The olanzapine study involved 11 participants at the start of the olanzapine; one dropout resulted in a total of two men and eight women completing the study. One of the remaining male patients discontinued the drug for 2 weeks and was the only patient whose anxiety score increased. The PEP-HD study had a 25% dropout rate for motor symptomatic HD mutation carriers (11 patients and six caregivers). Six out of the 37 participants dropped out during the course of the 1-year intensive therapy study. All three of these studies were not controlled, randomized, or blinded. None of the studies conducted follow-up assessments, so it is unclear whether the improvements were sustained across time. Although the psychosocial interventions demonstrated improvements and potentially no harmful side effects, it is not clear which aspects of the programs may have helped decrease anxiety symptoms.
The only intervention that also examined premanifest HD carriers was the PEP-HD study,47 but it did not affect anxiety levels among this group. Two other studies57,58 involving manifest HD participants did not have a significant impact on anxiety. One trial57 examined the effect of ketamine in a double-blind study, but no significant change in anxiety was identified. The second trial58 was a randomized, double-blind, placebo-controlled study of pridopidine (ACR16), 50 mg/day. The HADS was used as a secondary measure, with the main focus on cognition, resulting in a nonsignificant trend toward improvement in anxiety after 4 weeks.
Discussion
This is the first systematic attempt to review the status of research into anxiety in HD. Although anxiety is frequently reported in HD, none of the articles identified in this review examined anxiety as the central theme of the study, confirming that anxiety is a relatively neglected area of research within HD. Results of all studies in this review revealed a wider range of prevalence (13%−71%) among manifest HD participants, compared with the range reported in a previous review of psychopathology in HD undertaken in 2007.7 This finding was partly due to one study that examined a cumulative prevalence for 3 years resulting in higher anxiety levels (71%) among manifest HD carriers.15 Also, since 2007, studies using DSM-IV criteria have been published resulting in lower prevalence rates of anxiety.
Although prevalence studies of premanifest HD expansion carriers showed a lower range of anxiety (0%−15%), this outcome appeared to be the result of nearly all of the studies using tight diagnostic criteria and some studies having very small sample sizes.28,29,31 When studies of premanifest HD carriers with the largest samples available were compared with the manifest group using a formal diagnosis, there was limited difference in prevalence between the groups. This finding is supported by studies that have compared anxiety across manifest and premanifest HD expansion carriers and have found no difference in symptomology and diagnosis.4,23,25 These findings would appear to indicate that psychiatric problems precede the development of the motor aspect of the disease,3,5 although the extent to which anxiety is more prevalent in HD carriers compared with the general (non-HD) population is still unclear. The range of total anxiety diagnoses in the larger studies of HD mutation carriers was 12.5%−16.5% for point prevalence to 12-month prevalence. These results may be compared with rates of the general (non-HD) population, as determined by a systematic review of 41 prevalence studies,50 where a best estimate of 10.6% was given (with a 95% confidence interval of 7.5%−14.3%) for total anxiety disorders, across a 1-year period. Hence, although anxiety disorders appear to be more prevalent in HD compared with the general population, this cannot be a definitive conclusion without studies involving greater numbers of HD participants. Specific formal anxiety disorders that appear more prevalent in HD are GAD (3.8%−9%) and PD (3.6%−7.7%), with prevalence rates that are higher than in the general population.50
The factors that make comparisons of anxiety prevalence difficult across studies are the different measures used, different disease stages of participants, and rather small sample sizes. Many studies of anxiety in HD have used measures that were designed specifically to assess the range of behavioral symptoms in HD, like the PBA-HD, but have limited items for the assessment of anxiety. Hence, the full range of symptoms associated with anxiety problems may not be captured, along with the different anxiety disorders. Some of the measures used cover a range of anxiety symptoms (e.g., STAI, HADS), but their psychometric properties have not been fully explored for use in HD. DSM criteria may be the gold standard used to assess psychiatric diagnoses,7 but there may also be limitations due to the co-occurrence of somatic and cognitive problems and reduced insight into HD, particularly in the more advanced stages of the disease.51 Results from this review would indicate a requirement for more comprehensive measures of anxiety that are both valid and reliable for use within HD.
The results of this review indicate that it is still unclear whether HD expansion carriers experience more anxiety than verified noncarriers with an a priori 50% risk, which is an interesting area of research, as it assists in our understanding of whether the psychosocial factors of being in an HD family and being at risk for HD contribute to the development of anxiety. However, two recent studies with larger sample sizes than previous studies indicated that premanifest carriers reported more anxiety symptoms than noncarriers,3,4 suggesting that there may be factors specific to having the disease that underlie anxiety in HD. Many noncarriers may be anxious about their future before they have taken the predictive test, but it would appear from the research to date that even when they have received a negative test result, there is no difference in anxiety among those with a positive and negative result up to 5 years after testing.45 The possibility exists that as HD expansion carriers become closer to the onset of motor symptoms, anxiety increases, but so far the evidence does not support this.4,30 More research is required with larger sample sizes.
A number of variables have been examined in relationship to anxiety in HD. Anxiety is associated with depression in HD, although this relationship requires further elucidation because frequently both conditions have been examined together as a cluster of symptoms. Studies of temporal patterns of anxiety and depression in the general population have revealed that although there is evidence of a bidirectional relationship, anxiety disorders often predate depression.52 Importantly, anxiety has been found to have an independent association with suicidal ideation in HD, although it was not found to be a predictor across a 4-year longitudinal study.43 However, given that suicide is strongly related to depression and that anxiety may be a risk factor for depression, it is crucial that anxiety be identified and treated effectively within this population. Despite limited evidence, anxiety also appears to be related to QoL34 and aspects of functional ability,35 which would further confirm that this is an important area to address within HD.
Anxiety is also associated with irritability and agitation. This may be due to a number of different explanations such as anxiety arising in response to interpersonal difficulties created by irritability, that both conditions arise as a result of pathological changes associated with HD, or that anxiety arises from cognitive overload and leads to irritability in a person who lacks social cognition or who is impulsive. It would be useful to explore these relationships further.
In contrast, no relationships between anxiety and apathy, CAG repeat length, cognitive ability, motor performance, or disease progression were found. These findings indicate that anxiety does not follow the trajectory of the disease.8,15 It has been postulated that the peak of anxiety symptoms at around stage 2 of the disease may be indicative of changes that are occurring in the patient’s life associated with increasing loss of independence and/or an increase in pathological changes in the basal ganglia.8 Pain was found to independently predict anxiety in HD, and this area is worthy of further exploration in future studies. So far, there is limited research into the sociodemographic factors that might be associated with anxiety in HD, but no relationship with gender or age has been found.
Regarding the literature on interventions for anxiety in HD, this review identified little evidence of well-designed studies that demonstrated an improvement in anxiety in HD after a medical or psychological intervention. Three interventions have yielded promising results: 1) olanzapine; 2) a psychological intervention; and 3) an intensive multidisciplinary program, but none of these methods were controlled or randomized. All of these interventions were not specifically addressing anxiety but were wider studies examining a number of HD symptoms. There is a need for more robust intervention studies that are randomized, controlled, and blinded with a larger number of participants. A single case study of cognitive-behavioral therapy for a premanifest HD carrier revealed significant relief of anxiety,53 and wider trials of this approach could be undertaken among this group, which may enable carriers to develop coping strategies before significant cognitive deficits arise. As relationships between anxiety, illness perceptions, and coping styles are apparent in HD,21 more research in this area could help inform psychosocial interventions for HD.
This review had several limitations. First, our review was limited to articles published in English only and the studies included were relatively heterogeneous, which makes comparisons difficult. Some of the studies included in this review did not explicitly state how they determined whether or not participants were anxious. Most studies in this review excluded HD patients with severe dysarthria, dementia, and advanced-stage disease. The experience of anxiety in these groups requires further research, and suitable assessment methods will be needed. The use of caregiver report appears to show good usefulness in assessing psychopathology in advanced-stage HD.51 Many of the studies included had small sample sizes; hence, larger-scale studies are required. A variety of assessment tools were used in the studies, with many studies using tools limited to just one item on anxiety. The majority of studies included in this review did not report whether the sample was receiving treatment of anxiety or had done so in the past. Of the studies that did report medication use, there was variation in how such use was reported making comparisons difficult. Furthermore, there is also the possibility that anxiety may have resulted as a side effect of psychotropic medication or that medication-induced akathisia may have been erroneously labeled as anxiety.54
The majority of studies of anxiety in HD have been restricted to psychometric measures. Future studies may wish to pursue other paradigms such as physiological or neuroimaging measures. The extent to which various (epi)genetic, organic, and environmental factors contribute to anxiety in HD is still unknown. Further research may help determine whether the symptoms are associated with the pathology of the disease itself or whether familial and other psychosocial factors contribute to the development of anxiety. Research examining the onset of anxiety in HD would enable a better understanding of critical points of risk for patients and also premanifest HD expansion carriers. Given the extent of anxiety symptomology found among HD expansion carriers, it is important that an evidence base is established regarding interventions that can be used to treat anxiety in HD. We hope that this review will provide a basis from which future studies may increase our understanding of this often debilitating, but neglected aspect of HD.
1 : Huntington’s disease. Lancet 2007; 369:218–228Crossref, Medline, Google Scholar
2 : Huntington's disease: A disorder of families. Baltimore, MD, Johns Hopkins University Press, 1989Google Scholar
3 :
4 : Specific psychiatric manifestations among preclinical Huntington disease mutation carriers. Arch Neurol 2007; 64:116–121Crossref, Medline, Google Scholar
5 : Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry 2008; 79:874–880Crossref, Medline, Google Scholar
6 : Behavioral changes in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:219–226Medline, Google Scholar
7 : Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci 2007; 19:441–448Link, Google Scholar
8 : Depression and stages of Huntington’s disease. J Neuropsychiatry Clin Neurosci 2005; 17:496–502Link, Google Scholar
9 :
10 : Cognitive correlates of obsessive and compulsive symptoms in Huntington’s disease. Am J Psychiatry 2001; 158:799–801Crossref, Medline, Google Scholar
11
12 : A clinical study of patients with genetically confirmed Huntington’s disease from India. J Neurol Sci 2001; 190:73–78Crossref, Medline, Google Scholar
13 : Huntington disease: clinical care and evaluation. Neurology 1979; 29:1–3Crossref, Medline, Google Scholar
14 : Behavioural complaints in participants who underwent predictive testing for Huntington’s disease. J Med Genet 2002; 39:857–862Crossref, Medline, Google Scholar
15 : Longitudinal evaluation of neuropsychiatric symptoms in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2012; 24:53–60Link, Google Scholar
16 : The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
17 : Neuropsychiatric assessment of Gilles de la Tourette patients: comparative study with other hyperkinetic and hypokinetic movement disorders. Mov Disord 2001; 16:1098–1104Crossref, Medline, Google Scholar
18 : Neuropsychiatric aspects of Huntington’s disease. J Neurol Neurosurg Psychiatry 2001; 71:310–314Crossref, Medline, Google Scholar
19 : The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67:361–370Crossref, Medline, Google Scholar
20 : Palliative care and support for people with neurodegenerative conditions and their carers. Int J Palliat Nurs 2006; 12:368–377Crossref, Medline, Google Scholar
21 : Illness perceptions, coping styles and psychological distress in adults with Huntington’s disease. Psychol Health Med 2014; 19:169–179Crossref, Medline, Google Scholar
22 : Prediction of psychological functioning one year after the predictive test for Huntington’s disease and impact of the test result on reproductive decision making. J Med Genet 1996; 33:737–743Crossref, Medline, Google Scholar
23 : Cognitive and psychiatric characterization of patients with Huntington’s disease and their at-risk relatives. Neurol Sci 2002; 23(Suppl 2):S105–S106Crossref, Medline, Google Scholar
24 : Effect of CAG repeat length on psychiatric disorders in Huntington’s disease. J Psychiatr Res 2008; 42:544–549Crossref, Medline, Google Scholar
25 : Cross-sectional study on prevalences of psychiatric disorders in mutation carriers of Huntington’s disease compared with mutation-negative first-degree relatives. J Clin Psychiatry 2008; 69:1804–1810Crossref, Medline, Google Scholar
26 : Psychopathology in patients with degenerative cerebellar diseases: a comparison to Huntington’s disease. Am J Psychiatry 2002; 159:1306–1314Crossref, Medline, Google Scholar
27 : Psychiatric disorders in Huntington's disease: A 2-year follow-up study. Psychosomatics 2012; 53:220–229Crossref, Medline, Google Scholar
28 : Psychiatric symptoms and CAG repeats in neurologically asymptomatic Huntington’s disease gene carriers. Psychiatry Res 2001; 102:217–225Crossref, Medline, Google Scholar
29 : Psychiatric symptoms in neurologically asymptomatic Huntington’s disease gene carriers: a comparison with gene negative at risk subjects. Acta Psychiatr Scand 2002; 105:224–230Crossref, Medline, Google Scholar
30 : Psychiatric disorders in preclinical Huntington’s disease. J Neurol Neurosurg Psychiatry 2007; 78:939–943Crossref, Medline, Google Scholar
31 : A controlled psychiatric study of individuals at risk for Huntington’s disease. Br J Psychiatry 1994; 165:500–505Crossref, Medline, Google Scholar
32 : Psychiatric symptoms do not correlate with cognitive decline, motor symptoms, or CAG repeat length in Huntington’s disease. Arch Neurol 1996; 53:493–497Crossref, Medline, Google Scholar
33 : Neuropsychiatric assessment of patients with hyperkinetic and hypokinetic movement disorders. Arch Neurol 1998; 55:1313–1319Crossref, Medline, Google Scholar
34 : Mood and quality of life among people with progressive neurological illness. Int J Clin Health Psychol 2009; 9:21–35Google Scholar
35 :
36 : Determinants of irritability in Huntington’s disease. Acta Neuropsychiatr 2011; 23:309–314Crossref, Medline, Google Scholar
37 : Apathy is not depression. J Neuropsychiatry Clin Neurosci 1998; 10:314–319Link, Google Scholar
38 : Behavioural problems in Huntington’s disease using the Problem Behaviours Assessment. Gen Hosp Psychiatry 2008; 30:155–161Crossref, Medline, Google Scholar
39 :
40 :
41 :
42 :
43 : Suicidal ideation in a European Huntington's Disease Population. J Affect Disord 2013; 151:248–258Crossref, Medline, Google Scholar
44 : Psychological functioning before predictive testing for Huntington’s disease: the role of the parental disease, risk perception, and subjective proximity of the disease. J Med Genet 1999; 36:897–905Medline, Google Scholar
45 : Psychological distress in the 5-year period after predictive testing for Huntington’s disease. Eur J Hum Genet 2003; 11:30–38Crossref, Medline, Google Scholar
46 : Emotional and functional impact of DNA testing on patients with symptoms of Huntington’s disease. J Med Genet 1995; 32:516–518Crossref, Medline, Google Scholar
47 : The Patient Education Program for Huntington’s Disease (PEP-HD). J Huntingtons Dis 2012; 1:47–56Medline, Google Scholar
48 : Short-term effects of olanzapine in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:69–72Medline, Google Scholar
49 : Effects of a one year intensive multidisciplinary rehabilitation program for patients with Huntington's disease: a prospective intervention study. PLoS Curr 2013;5Google Scholar
50 : Prevalence and incidence studies of anxiety disorders: a systematic review of the literature. Can J Psychiatry 2006; 51:100–113Crossref, Medline, Google Scholar
51 : Measurement of psychopathology in Huntington’s disease: the critical role of caregivers. J Nerv Ment Dis 2010; 198:329–333Crossref, Medline, Google Scholar
52 : Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry 2010; 67:47–57Crossref, Medline, Google Scholar
53 : Cognitive-behavioural therapy with a Huntington’s gene positive patient. Patient Educ Couns 2003; 49:133–138Crossref, Medline, Google Scholar
54 ;
55 : Neuropsychological characteristics of Huntington’s disease carriers: a double blind study. J Med Genet 1995; 32:600–604Crossref, Medline, Google Scholar
56 : Predictive testing for Huntington’s disease: risk perception, reasons for testing and psychological profile of test applicants. Genet Couns 1995; 6:1–13Medline, Google Scholar
57 : Cognitive, behavioral, and motor effects of the NMDA antagonist ketamine in Huntington’s disease. Neurology 1997; 49:153–161Crossref, Medline, Google Scholar
58 : Efficacy and safety of the dopaminergic stabilizer Pridopidine (ACR16) in patients with Huntington’s disease. Clin Neuropharmacol 2010; 33:260–264Crossref, Medline, Google Scholar