Suicidal Behavior: A Distinct Psychobiology?
Over 800,000 suicide-related deaths are reported around the world every year, with one person committing suicide every 40 seconds (1). Extensive work aimed at improving suicide prevention has yet to deliver objective tools for better assessment and management of suicide risk. One of the hurdles has been a lack of full understanding of the underlying biological manifestations that lead to suicide. In order to conceptualize this complex phenomenon, the stress-diathesis model was proposed almost two decades ago and is still regarded as the most widely accepted hypothesis for understanding suicide. It describes suicidal behavior as the interplay between a stressor (e.g., an acute psychiatric condition or a negative psychosocial event) and an individual's vulnerability to experience suicidality. This vulnerability, or diathesis, potentially results from a genetic predisposition and epigenetic mechanisms related to early-life adversity (2, 3). Within this framework, substantial effort has been made to uncover the pathophysiology that would account for this diathesis. Thus far, findings are indicating that the suicide biological architecture consists of a distinct network of interrelated neural systems at play. Further study may unravel a holistic psychobiological foundation for suicidal behavior. This in turn would add support to the latter as being a discrete psychiatric disorder, a point that was suggested in DSM-5 and will be discussed in more details later. The present article reviews the most salient systems involved in the neurobiology of suicidal behavior and the interactions between them.
Serotonergic System
Owing to its major role in mediating impulsive aggression and affective instability, serotonin has already been linked to multiple psychopathologies and is heavily involved in the biological mechanism of suicide (4). Earlier studies have established a robust association between low CSF 5-hydroxyindoleacetic acid (5-HIAA), the main metabolite of serotonin and a reliable indicator of serotonin turnover, and suicide attempts, irrespective of psychiatric diagnoses (5). In contrast, suicide victims were found to have elevated tryptophan hydroxylase 2, the rate-limiting enzyme in serotonin synthesis in the brain, decreased serotonin transporter binding affinity, and increased serotonin neurons and concentration in the brainstem, perhaps compensating for low serotonergic activity (4). Taken together, these findings imply a defective transmission of serotonin (6). One proposed explanation is the elevated 5-HT1A autoreceptors binding affinity in the dorsal raphe nucleus of depressed suicide victims, which leads to decreased serotonin firing, and has been shown to predict more lethal suicidal behavior in a prospective cohort (7).
Hypothalamic Pituitary Adrenal (HPA) Axis
The HPA axis has also been heavily researched in relation to suicide, owing to its role in the stress-response system. To assess HPA axis dysregulation, the dexamethasone suppression test has been most commonly used, in which failure to suppress cortisol constitutes evidence for a hyperactive HPA axis. Several studies have found associations between dexamethasone suppression test non-suppression and a history of suicide attempts, higher hospitalization rate for suicide attempts, completed suicides, and a 14-fold higher risk for completed suicide (8–11).
Cortisol levels have also been used as a proxy measure for HPA axis activity. Both elevated and blunted levels have been associated with suicide attempts (12). Interestingly, a recent meta-analysis concluded that the direction of the association between cortisol and suicide attempts may be related to age (13), with higher levels in samples with a mean age under 40 compared with lower levels in samples with a mean age over 40 (13). While this finding may further elucidate the nature of the relationship between cortisol and suicide, it is important to remain cognizant of the marked methodological variability across studies included in this meta-analysis.
Neuroinflammation
Since the emergence of interferon-induced suicidal ideation and depression (14), the role of the inflammatory system in suicide has been garnering a lot of attention. Disturbances in cytokine levels have been associated with suicidality. The most consistent finding has been an elevated pro-inflammatory IL-6 level in blood and postmortem brain samples of individuals with suicidality compared with individuals without suicidality and healthy controls (15, 16).
Microgliosis, another indicator of neuroinflammation, has also been associated with suicide. In one study, a higher proportion of activated microglia and perivascular macrophage density was observed in the dorsal anterior cingulate white matter of postmortem brain samples from suicide deaths compared with matched nonpsychiatric deaths (17).
Additionally, large case-control studies found that omega-3 fatty acids, known for their anti-inflammatory properties, are low in individuals who attempted or completed suicide. Low levels also predicted future attempts in a prospective cohort (4). Although further studies are needed to confirm the association between omega 3 fatty acids and suicide, inflammation is nonetheless proving to be an area requiring further investigation.
Kynurenine Pathway
The kynurenine pathway, the main metabolic pathway for the degradation of tryptophan (see Figure 1), has made a relatively newer introduction to the suicide literature in the last few years. Quinolinic acid (QUIN), one of the main metabolites, is considered neurotoxic due to its activation of N-methyl-d-aspartate (NMDA) receptors, as well as the increased release of glutamate and inhibition of glutamate uptake, leading to glutamatergic neurotransmission overactivation. Another important metabolite, kynurenic acid (KYNA), is known for its neuroprotective, anticonvulsive, and antioxidant properties and acts mainly through NMDA, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors antagonism (18). A significantly higher level of QUIN in the CSF has been associated with suicide intent and attempts, with QUIN/KYNA ratio, otherwise known as the neurotoxic ratio, being 2-fold higher in suicide attempters compared with healthy controls. Furthermore, these abnormal QUIN levels were still elevated 2 years post-attempt. These findings, along with the fact that ketamine, an NMDA antagonist, has been demonstrated to have antisuicidal properties, make the kynurenine system worthy of further exploration (18).
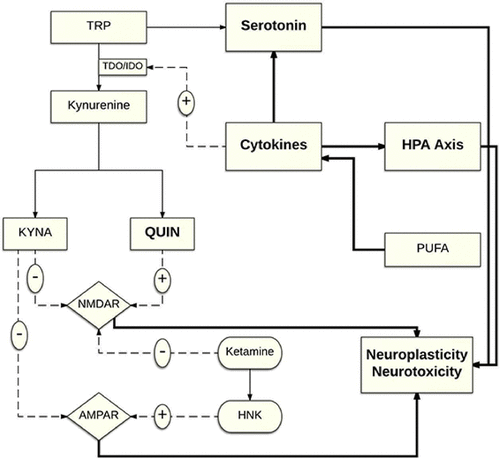
FIGURE 1. Interplay Between the Biological Systems Associated With Suicidea
a This figure highlights the interplay between the different neurobiological systems shown to be associated with suicidal behavior. AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; HNK: (2R,6R)-hydroxynorketamine; HPA: hypothalamic-pituitary-adrenal; IDO: indoleamine 2,3-deoxygenase; KYNA: kynurenic acid; NMDAR: N-methyl-d-aspartate receptor; PUFA: polyunsaturated fatty acid; TDO: tryptophan 2,3-dioxygenase; TRP: tryptophan; QUIN: quinolinic acid.
Neuroplasticity
Neuroplasticity refers to the processes by which the brain, through neurotrophic factors, undergoes functionally necessary adaptations in response to internal or environmental stimuli. Low levels of brain-derived neurotrophic factor (BDNF), a crucial mediator for neuronal survival and growth, and its receptor tropomyosin receptor kinase B or TrkB, have been found in suicide victims irrespective of the underlying psychiatric disorder (4), in some cases as a result of epigenetic changes (19). Reduced volume in crucial areas of the brain, such as the ventrolateral prefrontal cortex, has also been reported in patients with previous suicide attempt (20). These findings indicate increased neuronal loss and decreased neurogenesis in suicide.
Interplay of Biological Systems
As previously mentioned, a common suicide diathesis irrespective of psychiatric diagnosis has been suggested. This would require an underlying coherent pathophysiological mechanism that manifests itself into suicidal behavior (6). Therefore, a closer look at the dynamic and complex interplay between the different implicated systems is crucial to a better understanding of this machinery (Figure 1).
Neuroinflammation, by way of inflammatory cytokines, may contribute to the pathophysiology of suicide through different mechanisms, including stimulation of the HPA axis and dysregulation of the serotonin system (21, 22). Inflammatory cytokines also activate the enzyme indoleamine 2,3-deoxygenase, which catalyzes the initial step of the kyrunenine pathway (23). Although yet unstudied, this may theoretically contribute to decreased tryptophan metabolism into serotonin (16, 24). Additionally, downstream along the kynurenine pathway, QUIN's neurotoxic effects through NMDA receptor agonism might offer an explanation for the antisuicidal actions of NMDA antagonist ketamine. Moreover, in recent findings, ketamine's metabolite, (2R,6R)-hydroxynorketamine, has been shown to exert antidepressant effects through AMPA receptor activation (25). This in turn leads to BDNF release (26), which makes this pathway a plausible therapeutic target for antidepressants, as well as antisuicidal agents. Neurogenesis has also been observed to be significantly affected by different systems' dysfunctions, including dysregulation of the HPA axis and impaired serotonin transmission (6). Much research needs to be conducted to understand the precise nature of these interactions and how they fit into a coherent scheme.
Conclusions
The neurobiology of suicide is a highly complex phenomenon involving multiple interconnected neural systems. However, suicide research faces many limitations: small sample sizes, absence of animal models for suicidal behavior, phenotypic heterogeneity of suicidality, and exclusion of suicidal subjects from clinical trials for safety purposes. Additionally, the interplay between biological systems remains largely unstudied.
Considerations for the addition of suicidal behavior disorder as a separate DSM-5 diagnosis were made in an effort to resolve some of the issues mentioned above. Notably, suicidal behavior fulfills the criteria for diagnostic validity determined by Robins and Guze (27) and has been demonstrated to be a reliable diagnosis (28). Reframing our concept of suicide as a separate disorder may lead to a higher screening and detection of suicidal behavior in clinical practice and a common nomenclature for a well-defined phenotype. It may also help expand suicide research to analyses of large national or insurance data sets, providing a remedy to current small sample sizes (28).
With the advances in genetics, variants associated with psychiatric disorders are being detected at unprecedented rates, shedding light on novel biological systems that could explain the phenotype in question. Neuroimaging also constitutes a valuable tool for identifying the premise of the suicide diathesis on a circuitry level. As resources continue to be allocated to genetics and brain imaging research, the future for a better understanding of suicide and its prevention looks promising.
Key Points/Clinical Pearls
The stress-diathesis model for suicide refers to an individual's vulnerability to suicidal behavior in the context of psychosocial stress or an acute psychiatric condition.
The psychobiological nature of suicidal behavior consists of a distinct interplay between multiple neural systems, supporting the idea of suicidal behavior as a discrete psychiatric disorder.
Suicidal behavior satisfies the criteria for diagnostic validity and has been demonstrated to represent a reliable diagnosis.
1.
2. : Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry 1999; 156(2):181–189 Google Scholar
3. : The neurodevelopmental origins of suicidal behavior. Trends Neurosci 2012; 35(1):14–23 Crossref, Google Scholar
4. : Toward a biosignature for suicide. Am J Psychiatry 2014; 171(12):1259–1277 Link, Google Scholar
5. : Monoamine metabolites in CSF and suicidal behavior. Arch Gen Psychiatry 1981; 38(6):631–636 Crossref, Google Scholar
6. : The neurobiology of suicide. Lancet Psychiatry 2014; 1(1):63–72 Crossref, Google Scholar
7. : Positron emission tomographic imaging of the serotonergic system and prediction of risk and lethality of future suicidal behavior. JAMA Psychiatry 2016; 73:1048–1055 Crossref, Google Scholar
8. : HPA axis hyperactivity and attempted suicide in young adult mood disorder inpatients. J Affect Disord 2009; 116(1–2):117–120 Crossref, Google Scholar
9. : The dexamethasone suppression test as a predictor of suicidal behavior in unipolar depression. J Affect Disord 2004; 83(2–3):103–108 Crossref, Google Scholar
10. : Suicide and the dexamethasone suppression test in unipolar depression. Am J Psychiatry 1981; 138(8):1120–1121 Google Scholar
11. : The dexamethasone suppression test and suicide prediction. Am J Psychiatry 2001; 158(5):748–753 Link, Google Scholar
12. : A review of prospective studies of biologic predictors of suicidal behavior in mood disorders. Arch Suicide Res 2007; 11(1):3–16 Crossref, Google Scholar
13. : Cortisol levels and suicidal behavior: a meta-analysis. Psychoneuroendocrinology 2016; 63:370–379 Crossref, Google Scholar
14. : Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis C. Gen Hosp Psychiatry 2004; 26(3):237–240 Crossref, Google Scholar
15. : Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol Psychiatry 2015; 78(1):28–37 Crossref, Google Scholar
16. : The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology 2016; 63:296–310 Crossref, Google Scholar
17. : Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 2014; 42:50–59 Crossref, Google Scholar
18. : Kynurenine pathway metabolites and suicidality. Neuropharmacology 2017; 112(Pt B):324–330 Crossref, Google Scholar
19. : Alternative splicing, methylation state, and expression profile of tropomyosin-related kinase B in the frontal cortex of suicide completers. Arch Gen Psychiatry 2009; 66(1):22–32 Crossref, Google Scholar
20. : Prefrontal cortex markers of suicidal vulnerability in mood disorders: a model-based structural neuroimaging study with a translational perspective. Transl Psychiatry 2015; 5:e516 Crossref, Google Scholar
21. : Cytokine activation of the HPA axis. Ann N Y Acad Sci 2000; 917:608–617 Crossref, Google Scholar
22. : The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 2006; 31(10):2121–2131 Crossref, Google Scholar
23. : The kynurenine system and immunoregulation. J Neural Transm (Vienna) 2012; 119(2):197–209 Crossref, Google Scholar
24. : Role of inflammation in suicide: from mechanisms to treatment. Neuropsychopharmacology 2017; 42(1):271–283 Crossref, Google Scholar
25. : NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533(7604):481–486 Crossref, Google Scholar
26. : BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 2015; 18(1) Google Scholar
27. : Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry 1970; 126(7):983–987 Link, Google Scholar
28. : Suicidal behavior disorder as a diagnostic entity in the DSM-5 classification system: advantages outweigh limitations. World Psychiatry 2014; 13(2):128–130 Crossref, Google Scholar