Attention deficit hyperactivity disorder (ADHD) is the most commonly diagnosed behavioral disorder of childhood
(1). The prevalence of the disorder has been recently estimated to be 5%–10% of the general population
(1). The increase in diagnosis of ADHD seen over the past decade appears to reflect an increase in recognition of the disorder and has led to a dramatic increase in the prescription of methylphenidate
(2), the drug of choice in the treatment of ADHD
(3).
The therapeutic effects of methylphenidate are believed to be due to its ability to increase the synaptic concentration of dopamine
(4), which it does by blocking the dopamine transporters
(5). However, despite the widespread therapeutic use of methylphenidate, the levels of dopamine transporter blockade achieved at the doses used therapeutically in the treatment of ADHD are not known. In addition, while the pharmacokinetics of oral methylphenidate in blood have been widely investigated, there have been no studies of the pharmacokinetics of oral methylphenidate in the primate brain.
An evaluation of the levels of dopamine transporter blockade by oral methylphenidate is also of relevance vis à vis the abuse liability of methylphenidate
(6), since dopamine transporters are believed to be the target of cocaine’s reinforcing effects
(7). Dopamine transporter-blocking drugs, such as cocaine and methylphenidate, raise the extracellular concentration of dopamine in various brain regions including the nucleus accumbens, which is one of the brain structures associated with the reinforcing effects of drugs of abuse
(8).
With positron emission tomography (PET) and appropriate radiotracers, it is now possible to measure the levels of dopamine transporter occupancy achieved by drugs that block the dopamine transporter in human subjects reliably
(9) and also to evaluate drug pharmacokinetics directly in brain
(9). Using this strategy, we have shown a significant correlation between the magnitude of cocaine-induced dopamine transporter blockade and the self-reports for the “high” as well as a good temporal correspondence between the pharmacokinetics of cocaine at the dopamine transporter and the temporal course for the duration of the “high”
(9). Because we have shown that for intravenous cocaine to consistently induce a “high,” it has to block at least 60% of the dopamine transporters, we questioned whether methylphenidate, when given orally and at the doses used therapeutically, wouldachieves these levels of blockade.
To assess the level of dopamine transporter occupancy achieved by different doses of oral methylphenidate in human brain, we selected doses that covered the range used therapeutically for ADHD. The most frequent weight-adjusted doses for methylphenidate correspond to 0.3 to 0.6 mg/kg
(10). The behavioral and cardiovascular responses to methylphenidate were monitored during the PET studies. Parallel studies to measure the rate of uptake of oral methylphenidate in brain were performed in a baboon rather than in a human subject in consideration of the radiation dose to the oral mucosa. In addition, because of dosimetry considerations, we conducted the studies in adults rather than in children, for whom methylphenidate is most frequently prescribed.
RESULTS
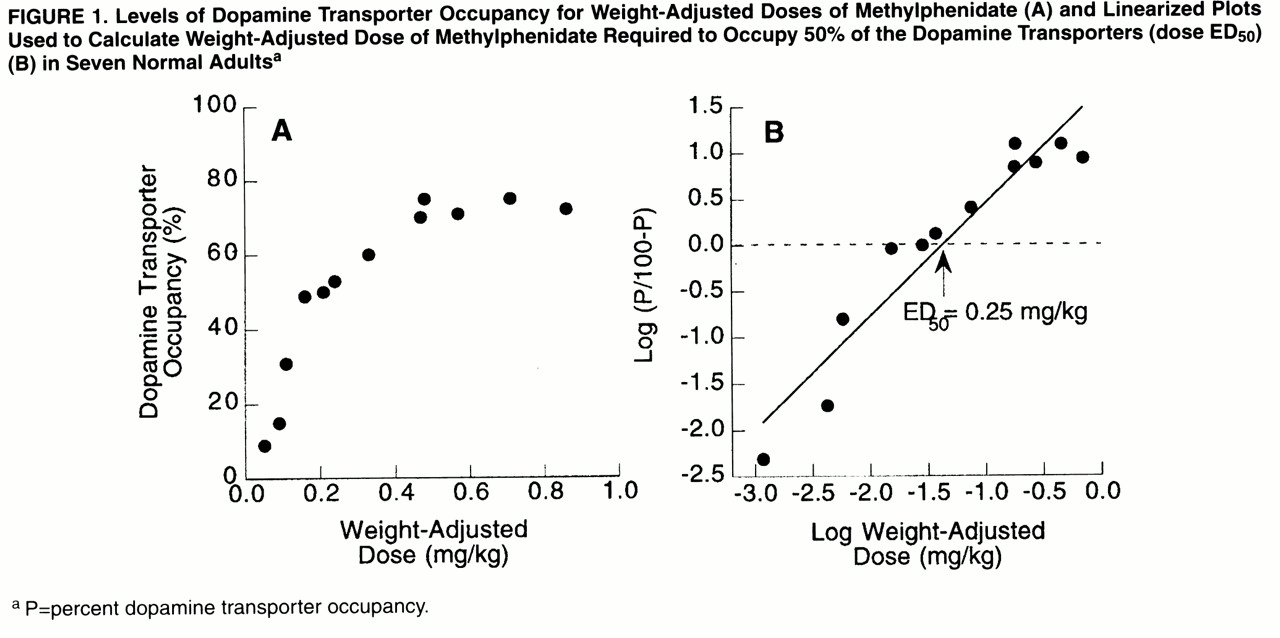
Oral methylphenidate produced a dose-dependent blockade of dopamine transporters: means=12% (SD=4%) for 5 mg, 40% (SD=12%) for 10 mg, 54% (SD=5%) for 20 mg, 72% (SD=3%) for 40 mg, and 74% (SD=2%) for 60 mg.
Figure 1A shows the levels of dopamine transporter occupancy for the weight-adjusted doses. The estimated dose required to block 50% of the dopamine transporter (ED
50 ) was 0.25 mg/kg (
figure 1B).
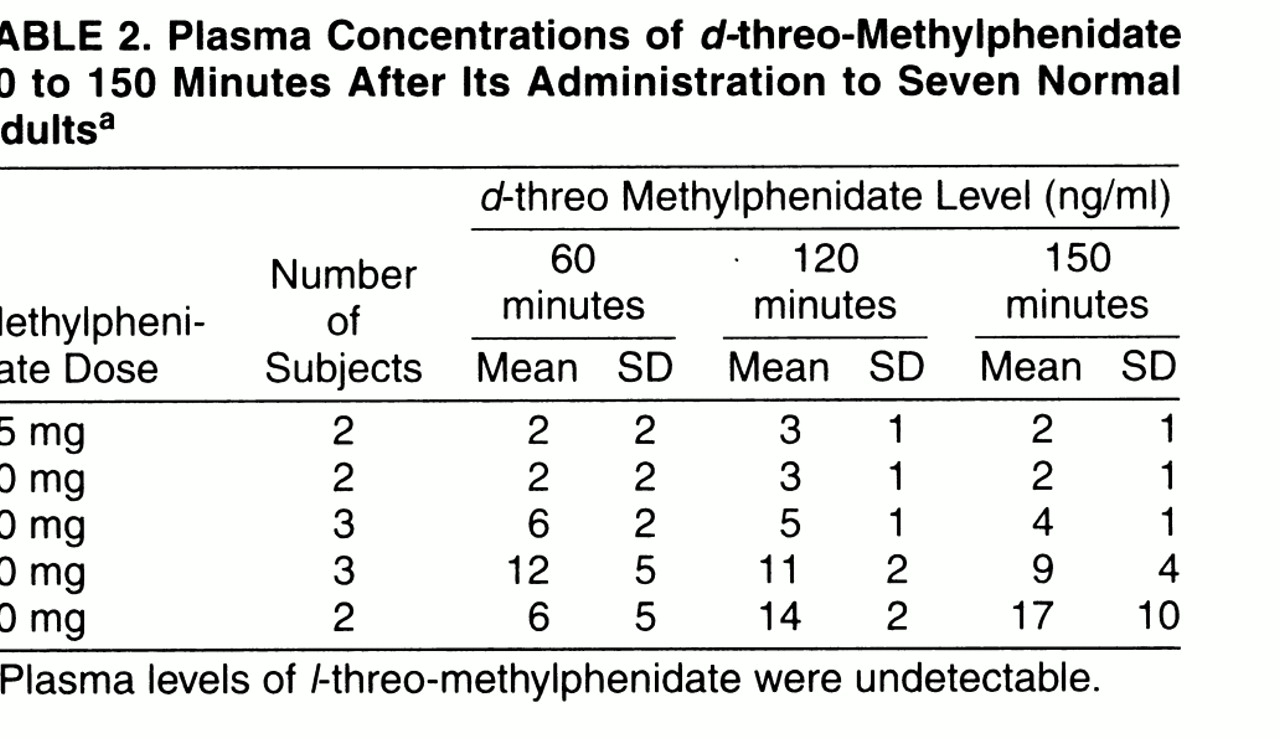
The plasma concentrations for
d-threo-methylphenidate are shown in
table 2;
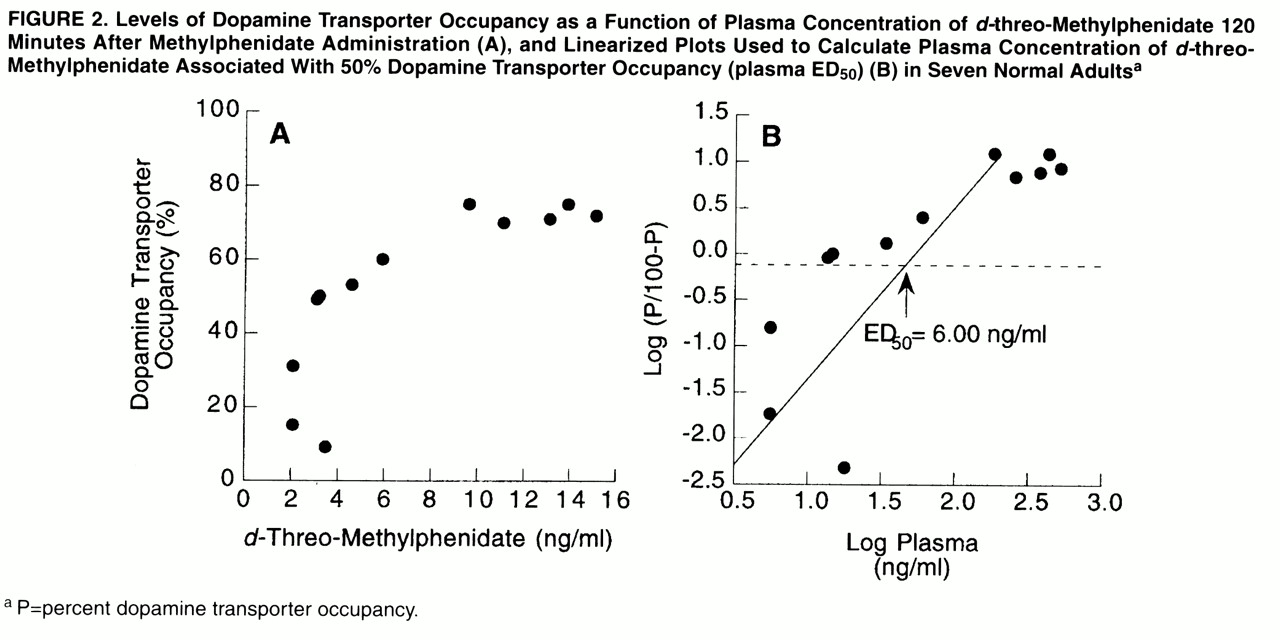
l-threo-methylphenidate was not detectable. Dopamine transporter occupancies were significantly correlated with the plasma concentration of
d-threo-methylphenidate at 120 minutes, which was the time when dopamine transporter measurements were made (r=0.80, df=11, p<0.002). The levels of dopamine transporter occupancy as a function of the concentration of
d-threo-methylphenidate at 120 minutes are shown in
figure 2A. The plasma concentration of
d-threo-methylphenidate, measured 2 hours after administration, that was associated with 50% blockade of the dopamine transporters was estimated to be 6 ng/ml (
figure 2B).
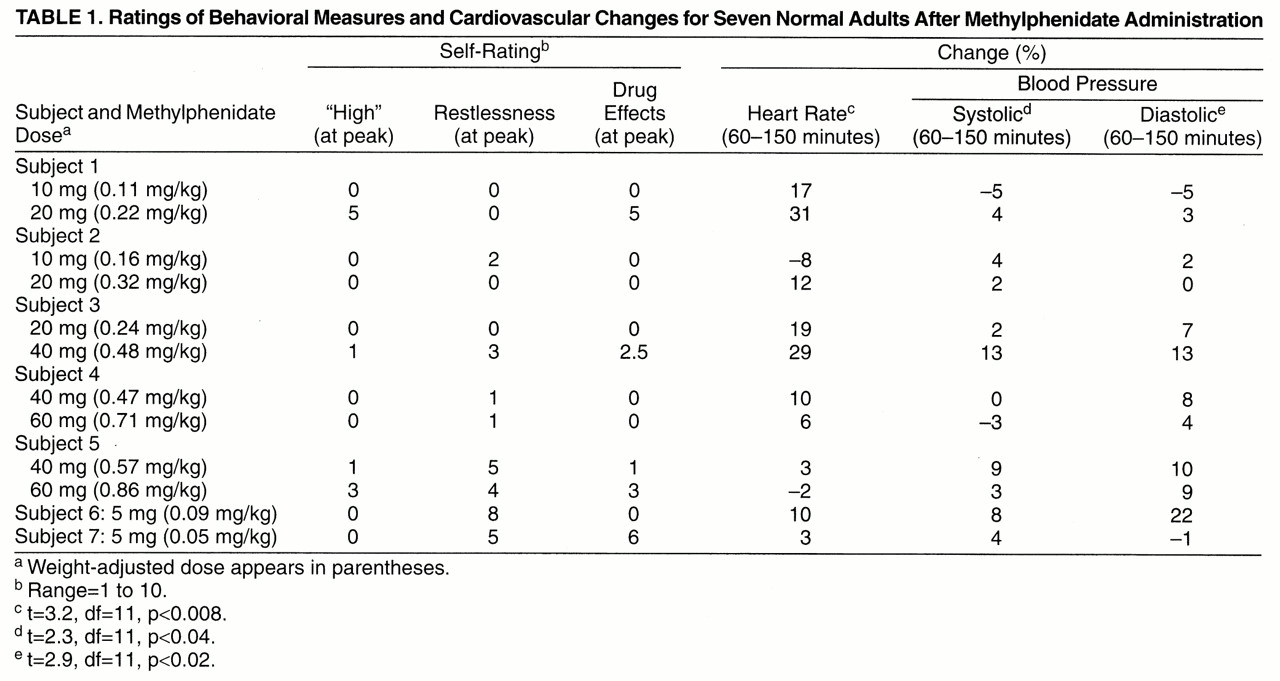
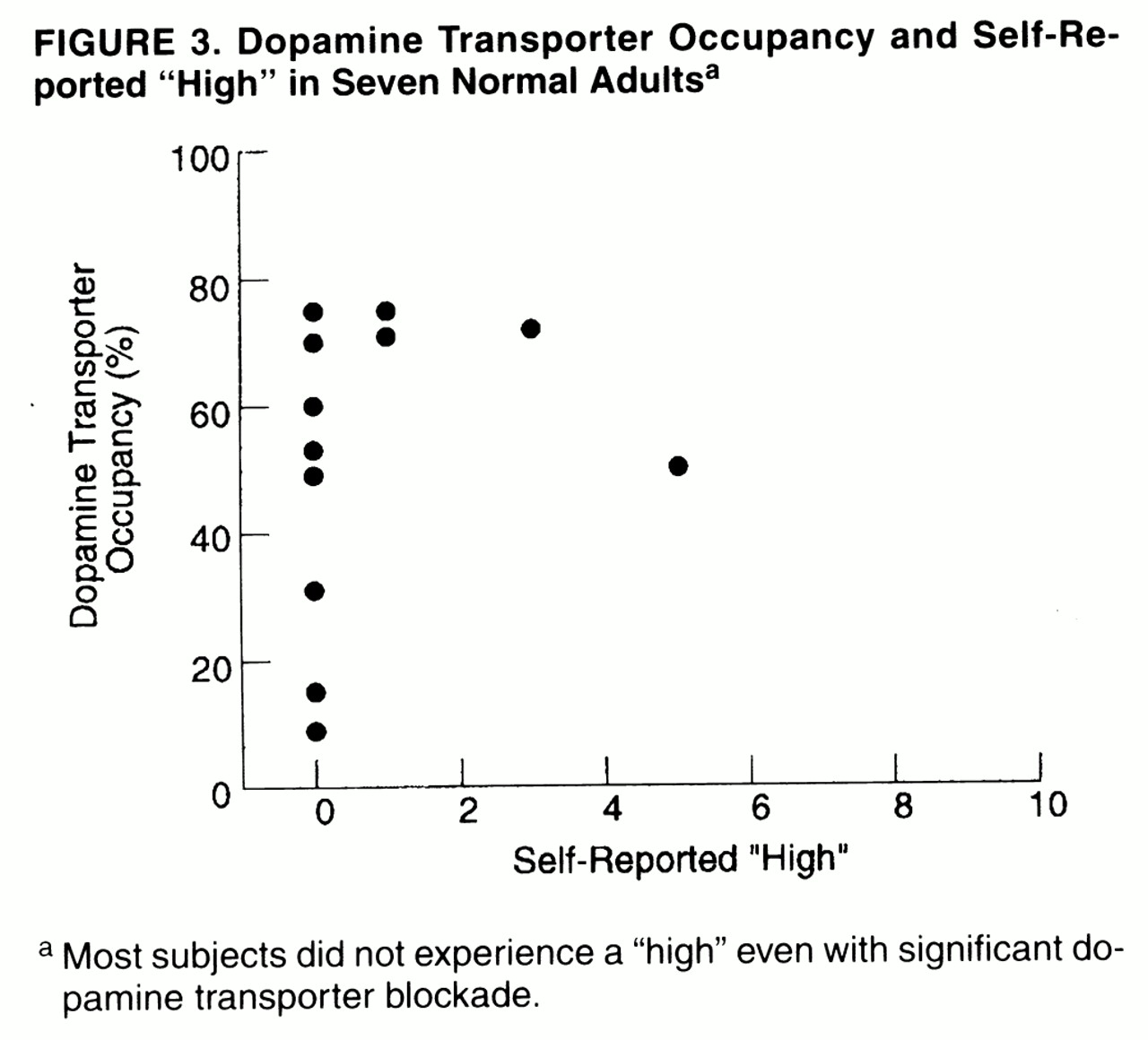
Only one of the subjects reported a moderate “high” for one of the methylphenidate doses, whereas the others reported no or minimal “high” (
table 1). Thus, even subjects who showed more than 60% dopamine transporter blockade did not report a “high” (
figure 3). Methylphenidate induced mild but significant increases in heart rate (placebo: mean=64%, SD=10%; methylphenidate: mean=71%, SD=7%) (t=3.2, df=11, p<0.008) and in systolic (placebo: mean=117, SD=14; methylphenidate: mean=120, SD=12) (t=2.3, df=11, p<0.04) and diastolic (placebo: mean=69, SD=5; methylphenidate: mean=73, SD=4) (t=2.9, df=11, p<0.02) blood pressure (
table 1). Group sizes were too small to assess dose effects.
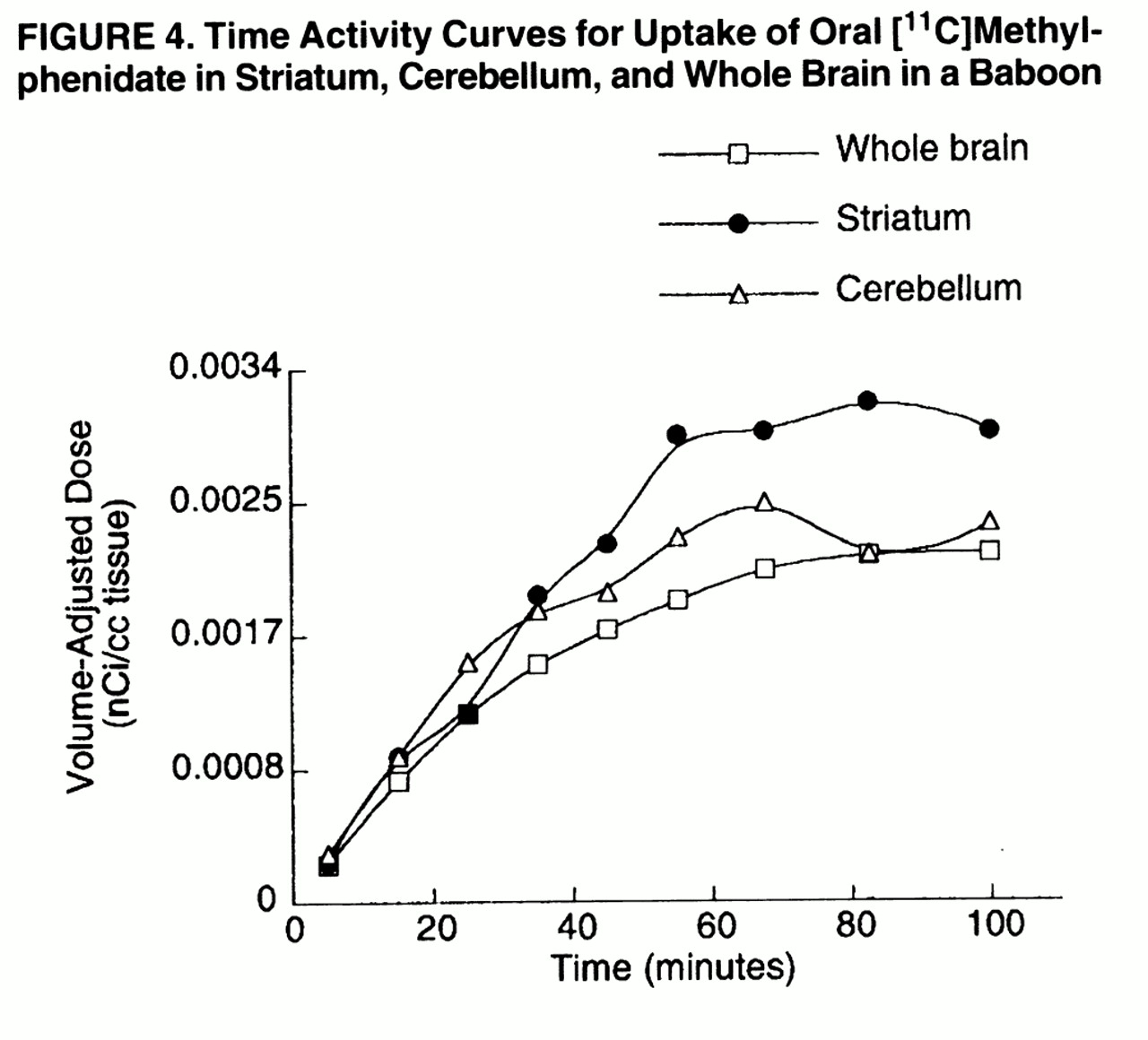
The baboon study showed that [
11C]methylphenidate did not reach peak concentration in brain until after 60 minutes of its administration (
figure 4). The short half-life of carbon-11 precluded an estimation of the clearance rate of [
11C]methylphenidate from brain.
DISCUSSION
This study shows that oral methylphenidate is very effective in blocking dopamine transporters. The ED
50 for dopamine transporter blockade by oral methylphenidate was estimated to be 0.25 mg/kg, which indicates that at the therapeutic doses used for ADHD (weight-adjusted doses of 0.3 to 0.6 mg/kg), methylphenidate is likely to occupy more than 50% of the dopamine transporters. This study also shows that the time to reach peak uptake of oral methylphenidate in brain was 60 minutes. This value corresponds well with the pharmacokinetics of methylphenidate reported in plasma, as well as with the time course for observing peak behavioral effects after therapeutic doses of oral methylphenidate
(21,
22). Unfortunately, because of the short half-life of carbon-11, we were unable to monitor the rate of clearance of methylphenidate in brain; such monitoring would have allowed us todetermine whether there was a correspondence between the duration of its behavioral effects and its presence in brain.
This study shows a correlation between plasma concentrations and dopamine transporter occupancy and suggests that plasma concentration of
d-threo-methylphenidate (active enantiomer) is a good indicator for monitoring methylphenidate delivery to brain. It also shows that plasma concentrations of 6 ng/ml of
d-threo-methylphenidate, for the measurements taken at 2 hours of drug administration, are associated with 50% blockade of the dopamine transporters. Since plasma levels in excess of 6 ng/ml at 2 hours of drug administration are reported in children with ADHD who receive methylphenidate doses that are clinically effective
(23), this suggests than greater than 50% dopamine transporter blockade may be required for therapeutic efficacy. A 0.25-mg/kg dose was estimated to correspond on average to a concentration of 6 ng/ml of
d-threo-methylphenidate in plasma, taken 2 hours after drug administration, which is consistent with plasma concentrations reported in adult subjects treated with these doses
(24). The variability in plasma methylphenidate concentrations among subjects, which is not unique to this study
(23), could reflect differences in drug absorption or metabolism or both among subjects as well as the small groups studied.
It had been postulated that oral methylphenidate has low reinforcing effects because of its rapid metabolism into ritalinic acid, a compound with low psychostimulant actions
(25). We had also hypothesized that oral methylphenidate, at the doses used therapeutically, would not induce a “high” because it would not achieve sufficient levels of dopamine transporter blockade. The results from this study do not support these hypotheses and suggest that variables other than poor efficacy at the dopamine transporter are responsible for the low reinforcing effects of oral methylphenidate. An important consideration in analyzing the reinforcing effects of drugs of abuse is the pharmacokinetics of these drugs, since the shorter the interval between intake and perceived effects of the drug, the greater the reinforcing effects of the drug
(26,
27). Of particular relevance is the manner of administration of these drugs, since it will affect their brain pharmacokinetics
(28,
29). In this study we showed that in the baboon brain, oral methylphenidate did not reach peak plasma concentrations until 60 minutes after its administration. This finding contrasts markedly with the fast rate of uptake seen in the baboon brain after intravenous administration of methylphenidate (8–10 minutes)
(30) or cocaine (4–6 minutes)
(14). In this respect it is worthwhile to note that while it has been shown that methylphenidate is abused by humans, such abuse occurs predominantly with intravenous rather than with oral administration
(6). Similarly, studies in nonhuman primates that have shown comparable levels of self-administration for methylphenidate and cocaine have used intravenous administration
(31,
32).
The dependency of the reinforcing effects of drugs on their rate of brain uptake could provide an explanation of why the subjects in this study did not report a “high” even when oral methylphenidate was given at doses that achieved levels of dopamine transporter blockade comparable to those of intravenous doses of cocaine
(9) or of intravenous doses of methylphenidate
(33), which induced a “high” under similar experimental conditions. These findings, in light of previous results showing that it is the rapid onset of blockade of the dopamine transporter, rather than continuous blockade, that is associated with the “high” induced by intravenous methylphenidate
(34), suggest that oral methylphenidate’s uptake in brain may be too slow to induce a “high.” In contrast to our results, others have reported mild reinforcing effects with similar doses of oral methylphenidate
(35). This may reflect differences in experimental conditions or the questionnaires used or both. In addition, the group sizes in the present study may have been too small to detect behavioral effects.
In interpreting the findings from this study, it is important to comment on the model used in the study to assess dopamine transporter occupancy (B
max/K
d=DV
STR/DV
CB–1). The distribution volume is an “equilibrium” measure representing the ratio of tissue radioactivity (metabolite corrected) to plasma radioactivity. Since it is an equilibrium property, it does not depend on blood flow. The distribution volume calculated for [
11C]cocaine with the graphical method has been shown to be the same as that obtained from a compartmental model approach and to be a stable measure that does not change with time
(14). Furthermore, estimates of dopamine transporter occupancy have been shown to be reproducible
(9) and to lead to similar measures whether one uses [
11C]cocaine or [
11C]
d-threo-methylphenidate as the dopamine transporter radioligand
(36). In interpreting the findings from this study, it is also important to realize that while the self-report of “high” has been shown to be a reliable and accurate predictor for drug self-administration in humans, it is not the only variable accounting for drug-seeking behavior
(37).
Limitations of this study include the fact that the studies were done in adults and not in children with ADHD and that the group size was small, which precluded an accurate analysis of methylphenidate’s behavioral effects. Furthermore, dopamine transporter measurements were made at 120 minutes and not at 60 minutes, when peak brain uptake was observed, so that peak occupancies may have been underestimated. In addition, while this study showed the levels of dopamine transporter occupancy obtained after various doses of methylphenidate, future studies are required to determine the levels of dopamine transporter blockade associated with methylphenidate’s therapeutic efficacy in ADHD.
This study measures for the first time the levels of dopamine transporter blockade achieved after therapeutic doses of oral methylphenidate. It shows that oral methylphenidate is very effective in blocking dopamine transporters and that at the doses used therapeutically for ADHD, it may block more than 50% of the dopamine transporters. It also shows that oral methylphenidate did not reach peak brain concentrations until 60 minutes after its administration. The slow rate of brain uptake of oral methylphenidate may explain the failure to induce significant self-reports of “high.” These results highlight the significant role that manner of administration has on the ability of methylphenidate to elicit a “high” and indicate that the “level,” as well as the “rate,” of dopamine transporter blockade contributes to the “high.” Further studies to elucidate the mechanism or mechanisms responsible for the “rate” dependency should be encouraged.