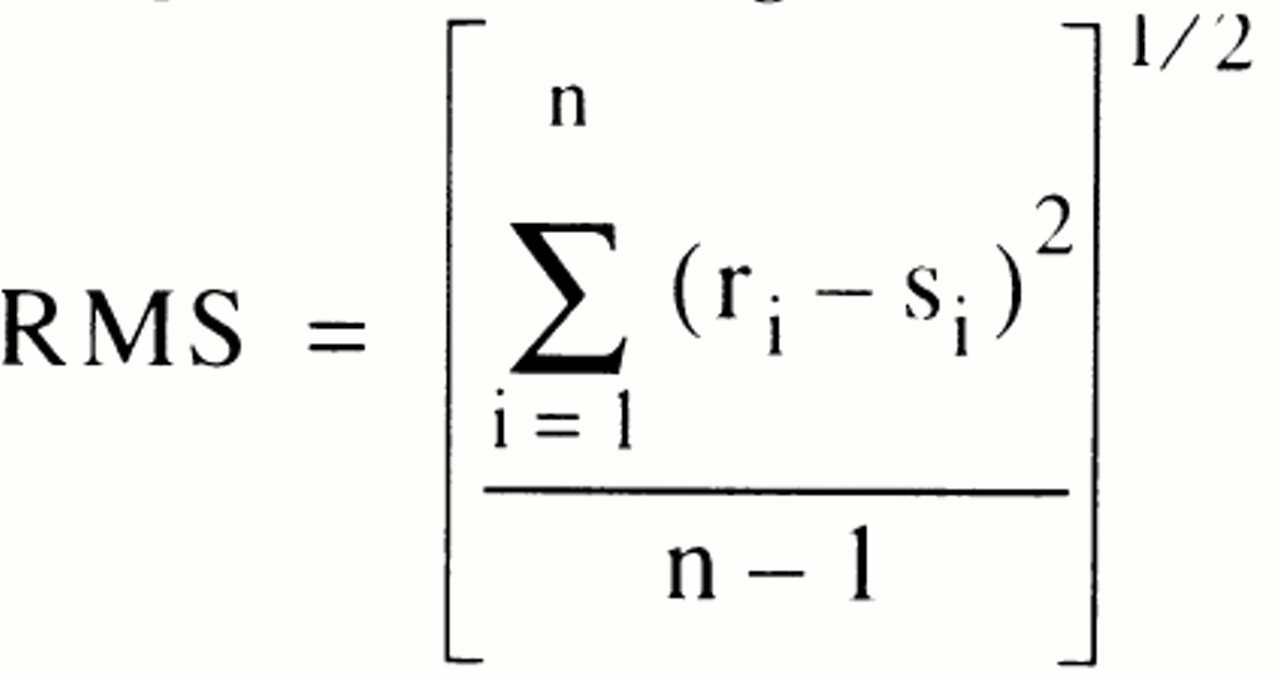Subjects
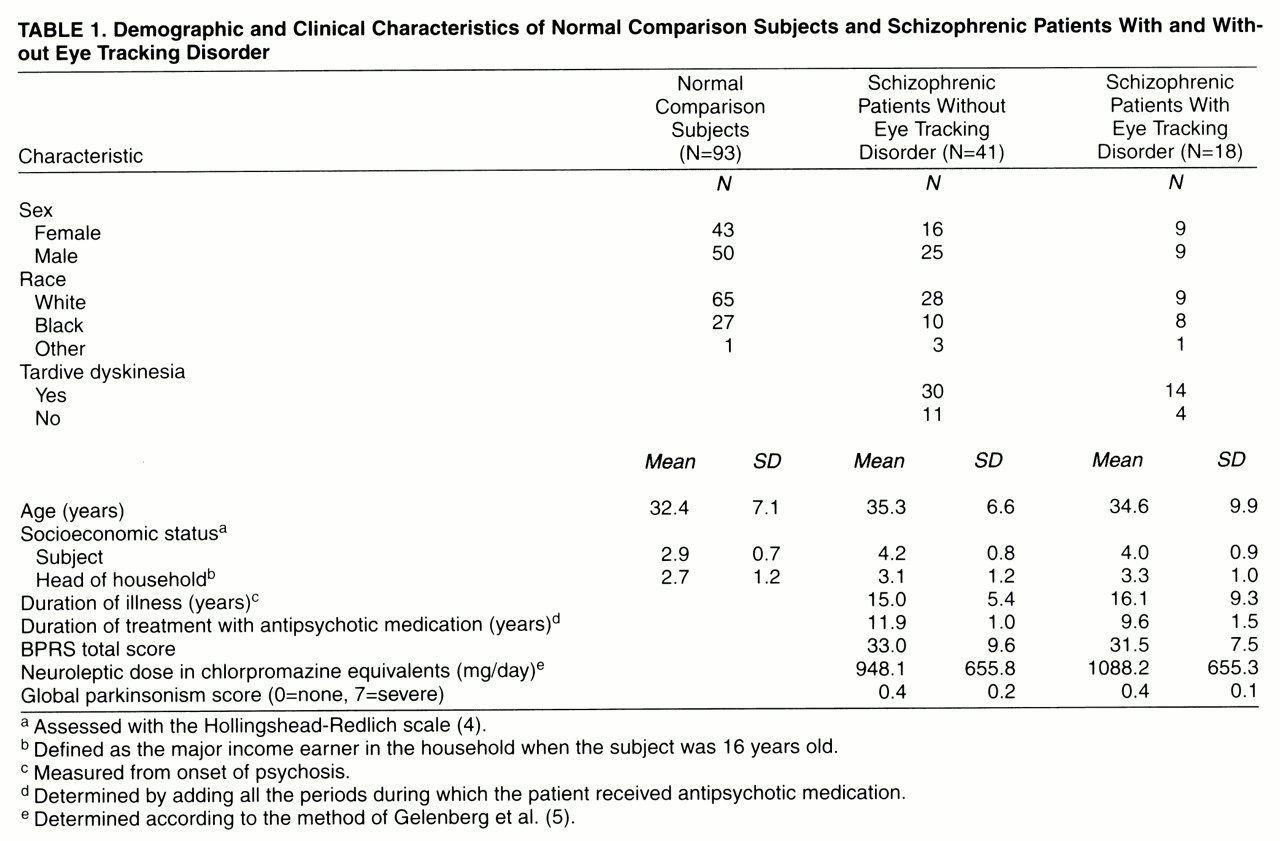
The participants in the study were 93 normal comparison subjects and 59 schizophrenic patients. The Structured Clinical Interview for DSM-III-R was administered to all subjects. Potential subjects were screened to exclude those with medical illnesses or taking medications known to adversely affect eye movements (including lithium and benzodiazepines). The socioeconomic status of each subject and the head of the household was obtained by using a previously developed scale
(4). Demographic and clinical characteristics of the subjects are shown in
table 1.
The normal comparison subjects were recruited from the general population through newspaper notices and flyers. They did not have any past or current DSM-III-R axis I or II disorder. They were reimbursed for their participation in the study.
Patients were recruited from outpatient and inpatient programs at the Maryland Psychiatric Research Center. All patients satisfied the DSM-III-R criteria for schizophrenia. All patients were clinically stabilized on antipsychotic medication regimens; some were taking antiparkinsonian medication. “Clinically stabilized” was defined as having taken the same antipsychotic medication for at least 4 weeks (usually longer) without a change in dose or significant change in clinical state as assessed by at least two treating clinicians, including the primary psychiatrist. The Brief Psychiatric Rating Scale (BPRS)
(6) was administered to each patient within 1 week of eye movement testing. Patients were assessed with the Maryland Psychiatric Research Center’s involuntary movement scale
(7) and diagnosed according to the research diagnostic criteria for tardive dyskinesia
(8) (
table 1). Tardive dyskinesia was diagnosed for 44 patients, and 15 were categorized as not having tardive dyskinesia.
The schizophrenic patients were classified into those with (N=18) and without (N=41) eye tracking disorder according to a previously described method
(9). In that study, mixture analysis showed that the distribution of position root mean square (RMS) error was best fit by a mixture of two normal distributions. That finding provided a rationale for determining a cutoff point that could be used to divide the patients into those with and without eye tracking disorder.
The patient subgroups and normal comparison subjects were compared to assess the presence of potential confounds. Chi-square tests were used for categorical variables, and independent t tests were used for continuous variables.
The three subject groups (normal comparison subjects, patients with eye tracking disorder, and patients without eye tracking disorder) did not differ significantly with respect to age, sex, race, or socioeconomic status of head of household (p>0.05 in all cases). The patients with and without eye tracking disorder did not differ significantly with respect to the subject’s socioeconomic status, duration of psychotic illness, duration of treatment with neuroleptic medication, neuroleptic dose in chlorpromazine equivalents, global parkinsonism score (from the involuntary movement scale) or total BPRS score within 1 week of eye movement testing (p>0.05 in all cases).
The patients with and without tardive dyskinesia did not differ significantly with respect to age, sex, or subject’s socioeconomic status (p>0.05 in all cases). They also did not differ significantly with respect to the presence or absence of eye tracking disorder (Fisher’s exact test, p=1.00; odds ratio=1.28). (The subjects were classified into the four cells as follows: both eye tracking disorder and tardive dyskinesia, 14 of 59 subjects [24%]; tardive dyskinesia without eye tracking disorder, 30 of 59 subjects [51%]; eye tracking disorder without tardive dyskinesia, four of 59 subjects [7%]; neither disorder, 11 of 59 subjects [19%].) The patients with and without tardive dyskinesia did not differ significantly with respect to scores on any of the subscales from the Neurological Evaluation Scale (to be discussed); for all cases, p>0.15 and d<0.4 (effect size
[10]).
After complete description of the study to the subjects, written informed consent was obtained.
Neurological Evaluation
All subjects were neurologically examined with the Neurological Evaluation Scale
(13), and this examination will be described only briefly here. The Neurological Evaluation Scale is designed to standardize the assessment of neurological impairment in schizophrenia. It consists of 26 items, which are divided into the following four subscales: 1) sensory integration, 2) motor coordination, 3) sequencing of complex motor acts, and 4) other neurological signs.
The sensory integration subscale measures the ability to integrate major sensory modalities, such as vision, hearing, and touch. It includes the following items: audiovisual integration (the ability to match a pattern of sounds to a visual diagram); stereognosis (the ability to identify common objects by touch); graphesthesia (the ability to identify numbers written on the skin); extinction (in response to bilateral and simultaneous somatosensory stimulation); and right/left confusion.
The motor coordination subscale measures the ability to coordinate movements. It includes the following items: tandem walk (walking toe to heel); diadochokinesis (rapidly alternating movements of the hands); finger-thumb opposition; and finger-nose test (touching one’s nose after closing one’s eyes).
The complex motor sequencing subscale measures the ability to rhythmically and regularly alternate hand positions. It includes the following items: fist-ring test; fist-edge-palm test; Ozeretski test; and rhythm tapping test, version B (generate a series of tapping sounds).
The “other neurological signs” subscale includes items that have been traditionally used or are of theoretical interest and that could not be clearly classified in any of the preceding categories. It includes the following items: adventitious overflow test; Romberg test; resting tremor; memory (remembering four words at 5 and 10 minutes); rhythm tapping test, version A (reproduce a series of tapping sounds); mirror movements; synkinesis (the ability to follow slowly moving object with the eyes only, not the head); convergence (ocular motor); gaze impersistence (in response to fixation of a peripheral, stationary target); and frontal release signs (glabellar, snout, suck, and grasp).
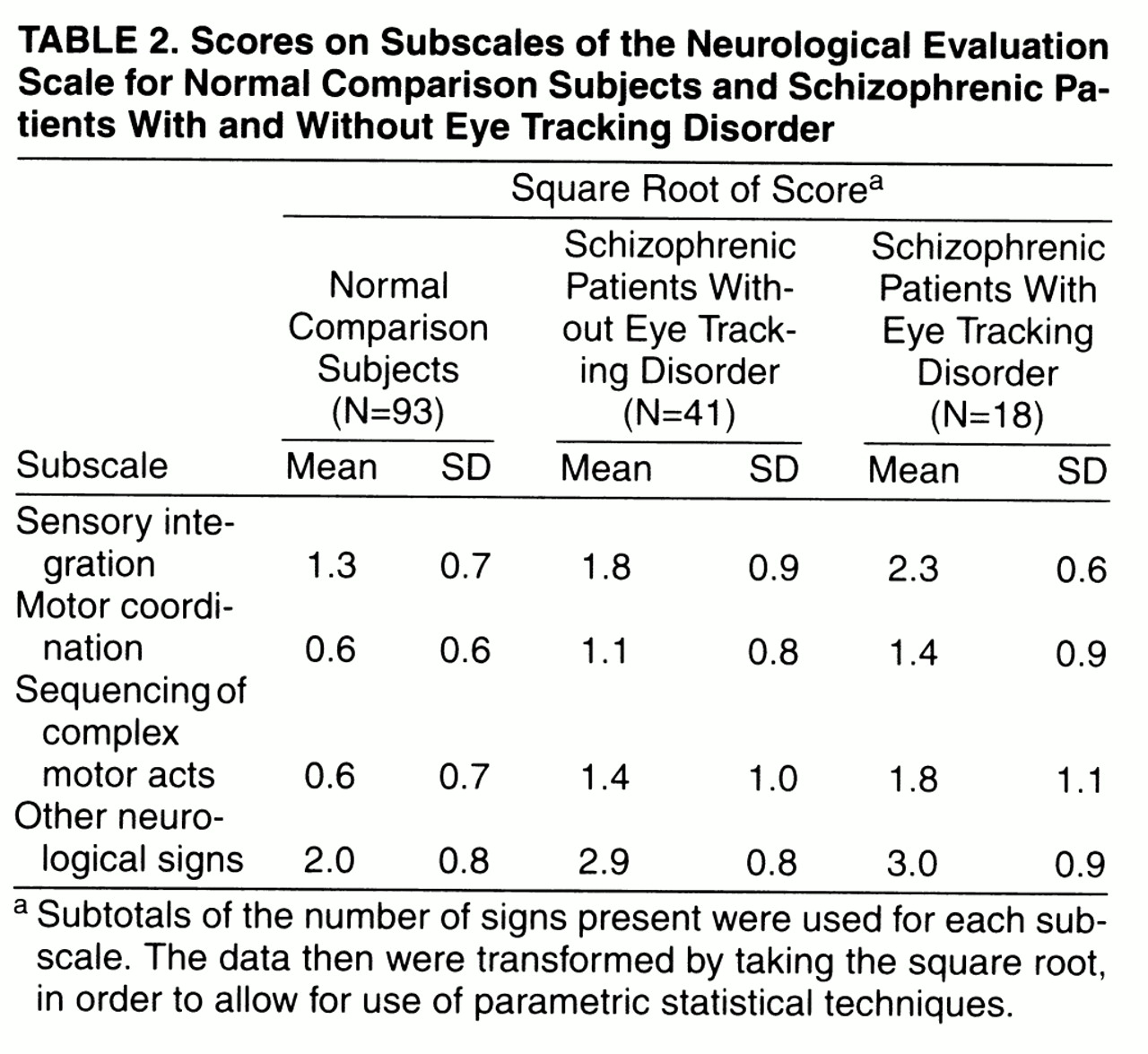
The neurological evaluations were completed by master’s- and doctoral-level clinicians who were trained in the use of the instruments and were blind to the results of the ocular motor analyses. Each sign was judged to be present or absent. The data were reduced by taking the subtotal of the number of signs present for each subscale. The interrater reliabilities for the subscales have been found to be at least moderately strong, with intraclass correlation coefficients ranging from 0.71 to 0.99
(13).
Statistical Analyses
The distributions of the data were examined for normality and homogeneity of variance. The variance of each of the four subscales of the Neurological Evaluation Scale differed significantly between groups (Brown-Forsythe test, df=2, 149, p<0.05 in all cases); the variances of the normal comparison group were larger than those of the patient subgroups. Therefore, a square-root transformation was applied to the data and was successful in removing the significant differences between groups (Brown-Forsythe test, df=2, 149, p>0.05 in all cases). Consequently, the transformed data were used in subsequent analyses, including between-groups comparisons and calculations of effect sizes.
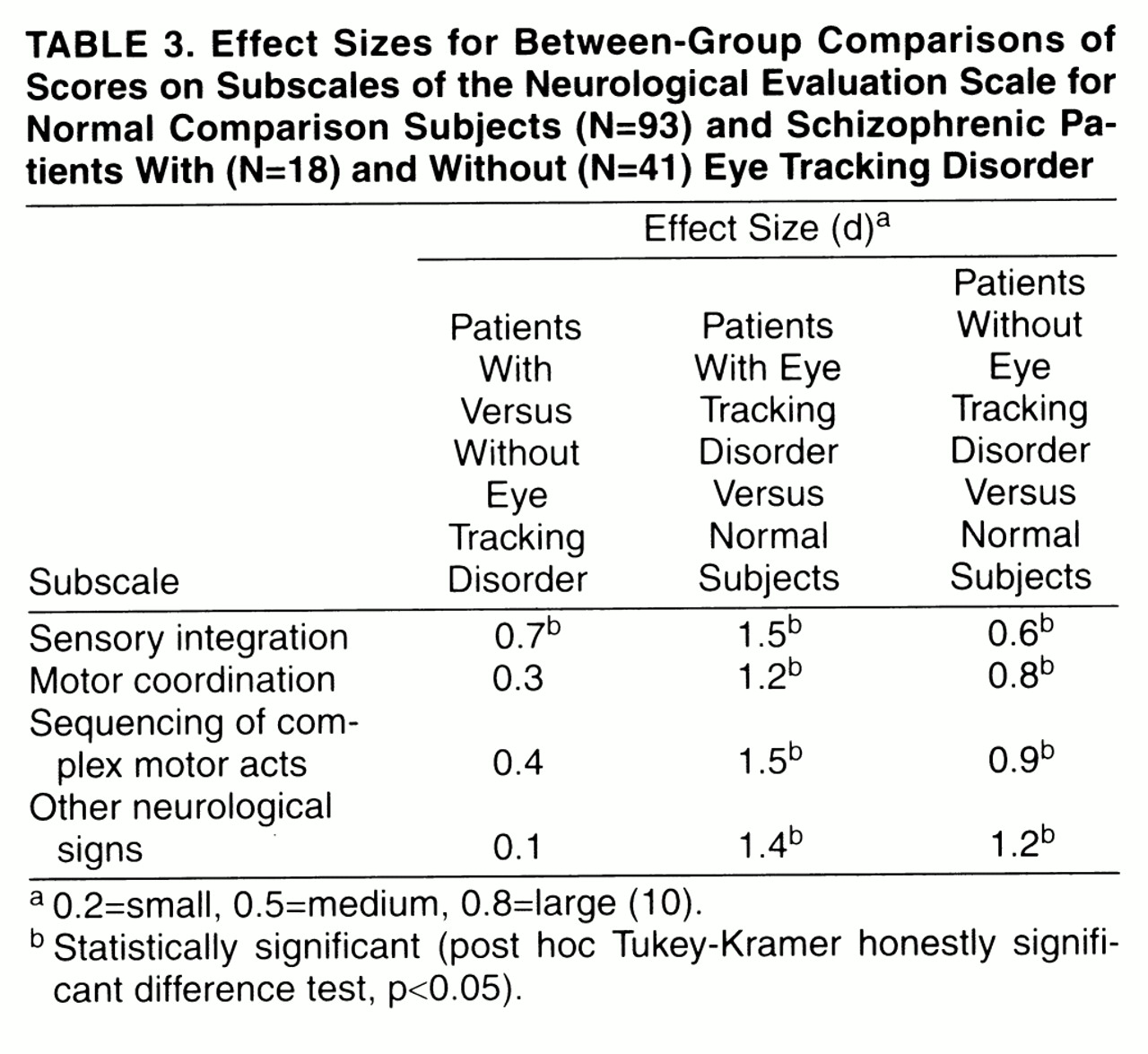
A multivariate analysis of variance (MANOVA) was used to test the hypothesis that the three groups (normal comparison subjects, patients with eye tracking disorder, and patients without eye tracking disorder) differed significantly with respect to the four Neurological Evaluation Scale subscales. Significant omnibus tests were followed up by post hoc Tukey’s honestly significant difference tests, in order to make the following comparisons: 1) patients with eye tracking disorder versus patients without eye tracking disorder, 2) patients with eye tracking disorder versus normal comparison subjects, and 3) patients without eye tracking disorder versus normal comparison subjects.