The hypothesis that a gene in the pseudoautosomal region of the sex chromosomes might confer vulnerability to schizophrenia has drawn mainly on two previous findings in the epidemiology of this syndrome: an increased prevalence of aberrations in the number of sex chromosomes among institutionalized subjects with schizophrenia
(1,
2) and the now 88-year-old observation that siblings affected with schizophrenia tend to be of the same sex more often than one would expect
(3). The validity of both findings has, however, been questioned: the first because of the only moderate strength of the association in the reverse direction (since most carriers of sex chromosome numerical aberrations do not exhibit schizophrenia
[4]) and the second mainly on methodological grounds
(5), namely, incomplete and biased ascertainment of affected sibling pairs in samples of limited size. We therefore addressed the issue of increased sex concordance in affected siblings in a sample of 1,942 sibships with at least two members treated for schizophrenia ascertained through a combination of three case registers with nationwide coverage in Finland. Since the same set of registers served for the identification of families multiply affected with schizophrenia for the purpose of molecular genetic linkage studies, any epidemiologic finding of an increased sex concordance in the sampled population would directly bear on our a priori chances of establishing a positive finding of linkage between schizophrenia and the pseudoautosomal region in this panel of families.
How would an increased concordance for sex in sibling pairs with schizophrenia link to a tiny region of the sex chromosomes at the tip of their short arms? In meiosis correct pairing of autosomal chromosomes and the two X chromosomes of females is ensured by sequence homology, and crossing-over followed by exchanges of small DNA stretches by recombination may occur anywhere along their full length. In the X and the Y chromosomes undergoing meiosis in a male, however, sequence homology is largely restricted to the aforementioned region, to which a single obligatory crossing-over is confined, whereas the subsequent sex-specific region is devoid of recombinational events. Thus, a vulnerability-conferring gene residing in the sex-specific stretch of the X chromosome of an affected male would be transmitted only to his daughters, and a locus on the Y chromosome only to his sons, leading to perfect sex concordance of affected siblings. However, transmission of schizophrenia from an affected father to both his sons and his daughters (i.e., sex discordance of affected siblings) is too common to be reconcilable with this particular location of a single disease-associated gene of major effect. By contrast, if a disease-associated locus were to reside in the pseudoautosomal region, the degree in excess of expectations to which sex concordance of affected offspring of an affected male proband is observed would follow a gradient depending on its location: the farther the locus from the junction toward the tip of the chromosome, the greater the chances of its being separated from sex-determining loci in the sex-specific region by the obligatory crossing-over
(6), and the lower the degree of observable increase of sex concordance in affected offspring of the male. In any case, transmission of a gene in the pseudoautosomal region from affected female probands would be expected to result in a 50:50 ratio of same-sex to opposite-sex affected offspring pairs (at least when equal numbers of affected male and female offspring are assumed).
Nongenetic mechanisms have been considered in explanation of previous findings of increased concordance for sex, namely, the tendency of same-sex sibling pairs to spend more time together than opposite-sex pairs, at least in childhood and adolescence. However, twin studies designed to gauge the influence of genetic and environmental factors on the susceptibility to schizophrenia assigned mostly a minor role to environmental factors, and among these the influence of shared environmental factors was poor compared with that of individual ones
(7). On the other hand, it is hard to conceive of any genetic mechanism other than involvement of the pseudoautosomal region to account for an excess in sex concordance, and any proof or refutation of the latter would directly enhance or challenge the validity of the former.
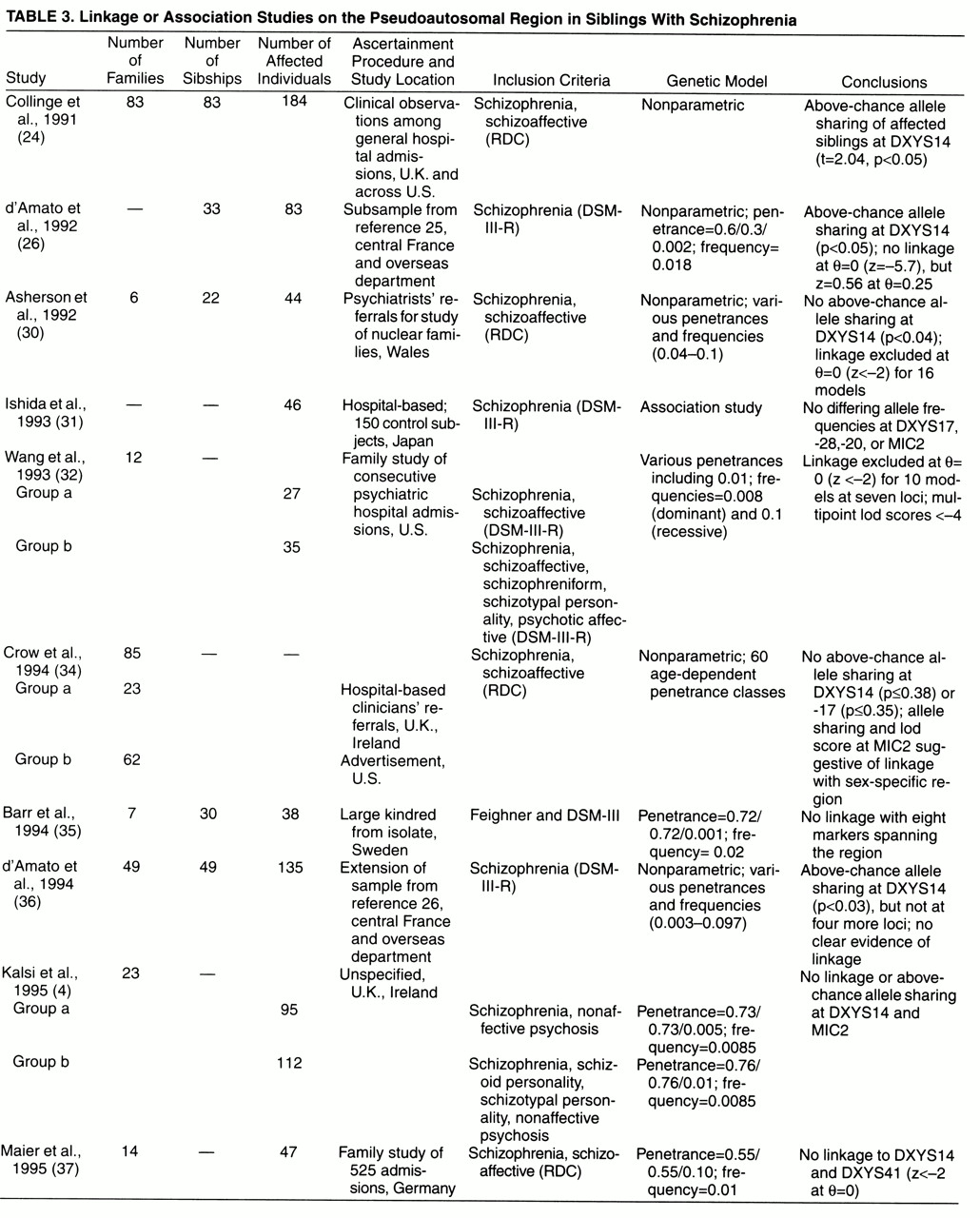
In an attempt to overcome methodological obstacles in historic samples
(3,
8-
17) as reviewed by Crow et al.
(18,
19), we tested in a nationwide sample 1) whether siblings with schizophrenia from multiply affected sibships were more often of the same sex, rather than opposite sexes, than expected from their sex ratio when their fathers but not their mothers were also affected with schizophrenia, 2) whether increased sex concordance extended to the nationwide sample irrespective of parents’ status (as might be expected if a positive effect from hypothesis 1 were strong enough), and 3) whether concordance for sex would not deviate from expectations in any direction in sibships in which the mother but not the father was affected.
METHOD
Through inquiry to three independent case registers with nationwide coverage of Finland, we identified a total of 29,124 individuals from the birth cohort of 1940–1969 who had been diagnosed with and treated for schizophrenia, as indicated by at least one of the following: 1) registration in the social insurance files as a recipient of a disability pension because of chronic schizophrenia, 2) registration in the national health insurance files as a recipient of cost-free antipsychotic medication for schizophrenia, and 3) a listing in the national hospital discharge register as having a discharge diagnosis of schizophrenia (according to ICD-8 from 1968 to 1986 and DSM-III-R since 1987). Persons in the first registry routinely undergo independent diagnostic evaluation by a psychiatrist in the process of applying for a disability pension, for which everyone between 16 and 65 years of age with a chronic course of schizophrenia who is not able to maintain himself or herself by regular work is eligible, independent of sex and prior employment status. Dropouts from the second register, who would at the same time be missing from the third, include the few never-hospitalized persons who seek treatment in a private practice setting rather than with the extensive national health system and thereby risk loss of reimbursement for health maintenance costs. Although exact figures are unavailable, we believe that these instances are rare among persons with chronic schizophrenia. As there are no privately funded inpatient units in Finland, coverage of ever-hospitalized patients by the third registry is complete.
Personal diagnostic interviews with standardized schedules are impossible to conduct for such a high number of subjects; therefore, the reliability of registered diagnoses was checked by independent best estimate
(20), with the use of DSM-III-R criteria, from all available medical records in a subsample of the probands
(21). Of 75 registered cases, 57 (76%) of the probands retained a DSM-III-R diagnosis of schizophrenia, and for a further 12 probands, the diagnosed disorder was one of the conditions that, by cosegregation with schizophrenia in family studies, have been shown to be part of a genetic schizophrenia spectrum (schizoaffective disorder in seven cases; schizoid personality disorder in two; and schizophreniform disorder, delusional disorder, and schizotypal personality disorder in one case each). However, six probands (8%) had diagnoses that did not fall within the schizophrenia spectrum (four with a major affective disorder—although three of them had a psychotic affective disorder, a condition sometimes considered to be a further extension of the schizophrenia spectrum—and one each with reactive psychosis and alcohol hallucinosis) and were thus wrongly included as cases of near-schizophrenia in the registers.
By means of the unique individual identification number every Finnish citizen receives upon birth, the identified cases—primarily independent observations—were cross-linked with the national birth register to establish nuclear families with several affected siblings, whose parents’ data could then be rechecked for affection status with the set of three case registers. In this way, a nationwide panel of affected sibships with one parent, both parents, or none affected was identified, corresponding to the closest-relative approach
(18) for determining lineality of transmission, but with the advantage of considering the same narrow disease category from the same data sources in the parents as in the probands.
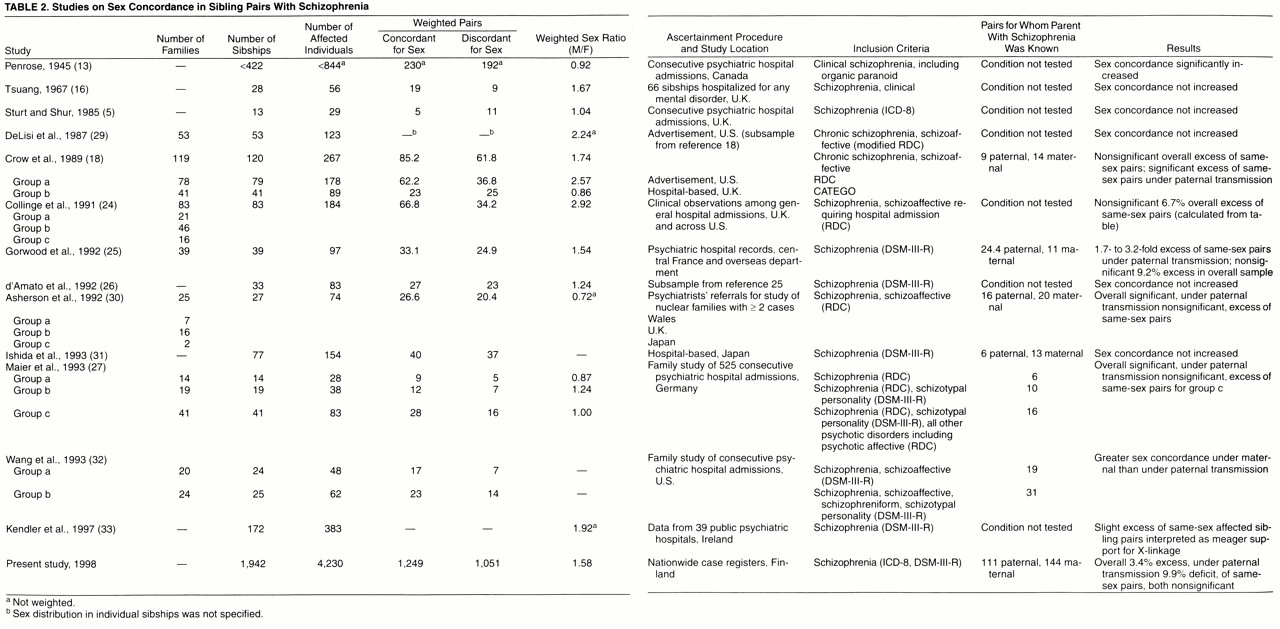
Previous studies considered sibships with more than two affected members as a single observation of sex concordance when all affected siblings were of the same sex and of sex discordance when at least one of them differed in sex from the others
(18). However, this sibshipwise strategy seems implausible, since it disregards the major instances of sex concordance in sibships where multiple affected members are of the same sex, but because of a single sibling of the opposite sex, the whole sibship enters the calculations only once as an observation of sex discordance. Most studies have therefore dissected those sibships with n affected members into all possible pairs (1+2+…+[n–1]=n[n–1]/2) and multiplied the resulting number of same-sex and opposite-sex pairs by 2/n to yield weighted pairs
(22). These were considered as independent observations, yet despite the correction factor, multiple pairs from within the same sibship still involve the same meioses, a bias that has until recently also been overlooked in linkage studies of allele sharing among affected siblings. We circumvent this problem by regarding each meiosis (instead of sibling pairs) as the actual independent unit of observation, thus making use of the information contained in multiply affected sibships jointly instead of separately. A full likelihood analysis
(23) is the appropriate strategy: the probability of the observed sex data is computed, given increased concordance for sex (with a parameter, δ, representing departure from the expected independence of sex of affected siblings) and also under the assumption of no deviation in sex concordance (null hypothesis: δ=1). The ratio of these probabilities indicates how much more likely increased sex concordance will be than the null hypothesis, given the observed data. The formal derivation of this likelihood analysis is as follows.
If we assume that the siblings are not correlated by sex, then the likelihood of a given sibship is given by
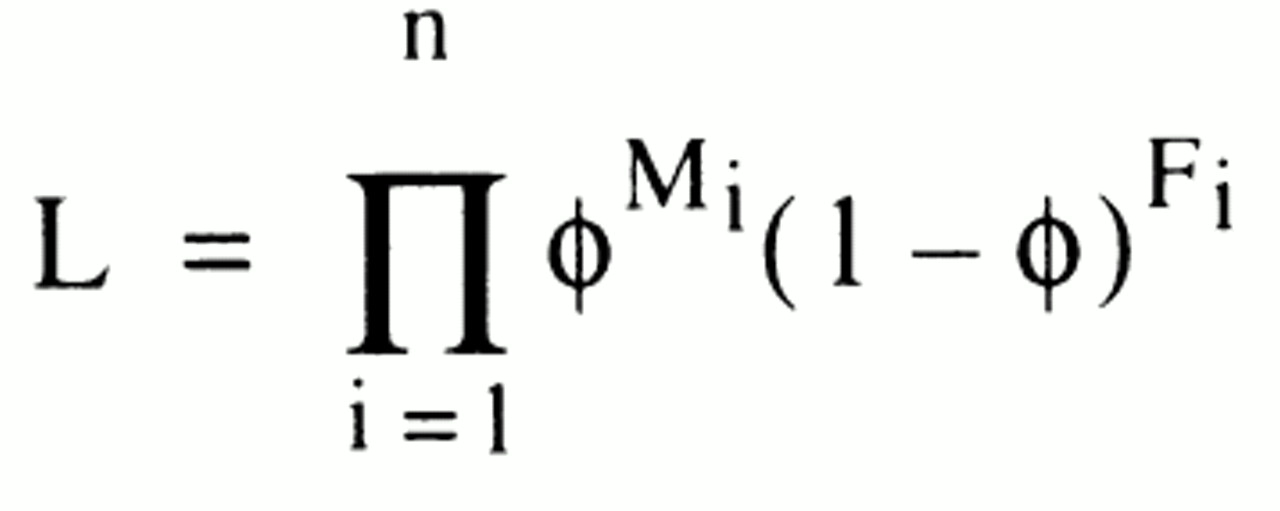
where ϕ is the probability of a random affected person being male, and the product is taken over all sibships, i. The maximum likelihood estimate of ϕ can be shown to be the binomial proportion
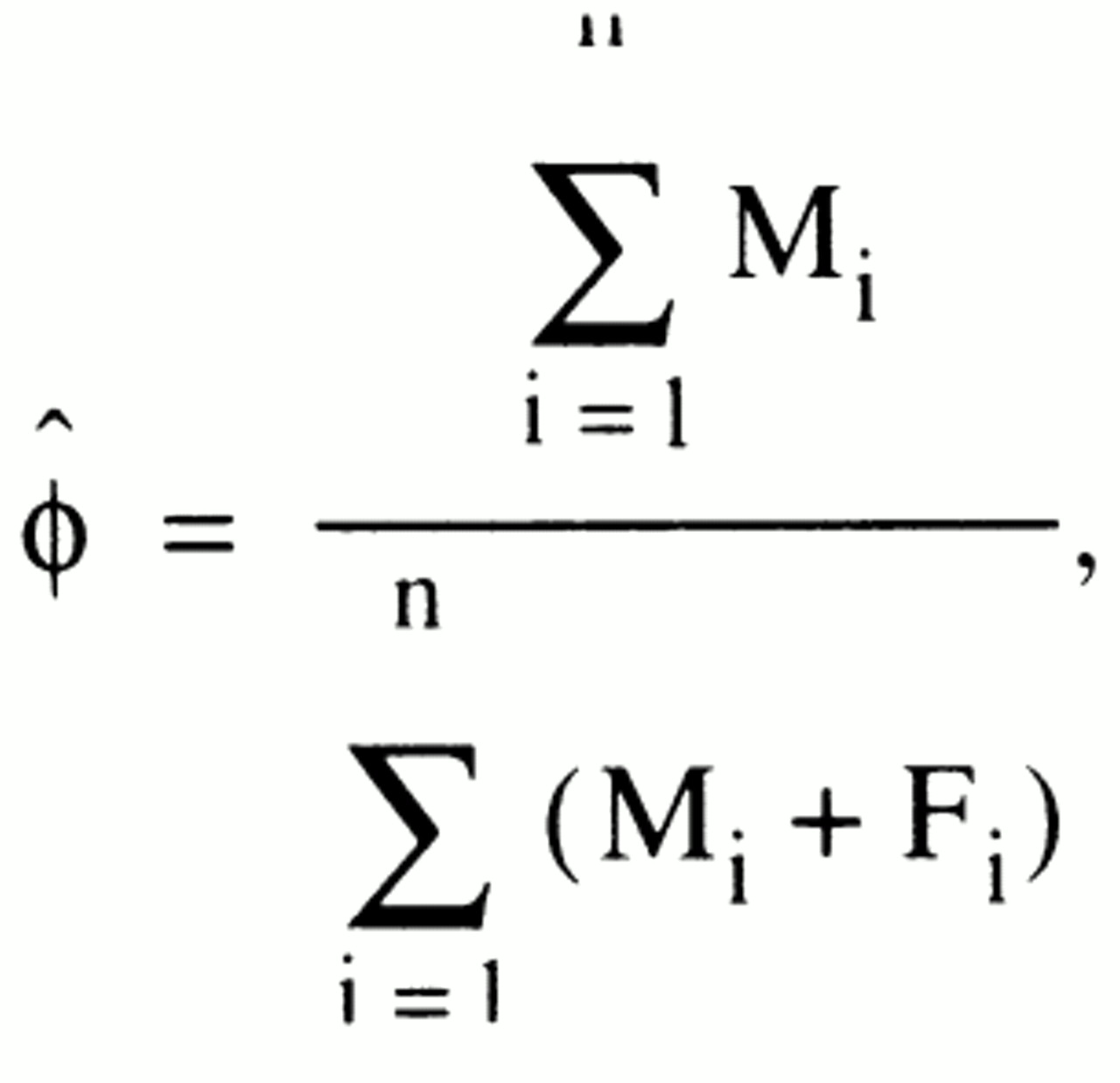
where M
i is the number of males in sibship i, and F
i is the number of females in the same sibship i. Under the alternative hypothesis, it is presumed that when the proband is male, there should be a reduced probability that remaining siblings are female, and thus the likelihood for a given sibship with a male proband is given as L
i,male=ϕ[δ(1–ϕ)]
Fi
[1–δ(1–ϕ)]
Mi
–1, and with a female proband the likelihood would correspondingly be L
i,female=(1–ϕ)[δϕ]
Mi
[1–δϕ]
Fi
–1. However, since the proband is typically unknown, one can determine the probability that a given sibship had a male proband as ψ=M
i / (M
i+F
i). Thus, the overall likelihood for a given sibship with an unknown proband would be L
i=ψL
i,male+(1–ψ)L
i,female, and when δ=1, this is identical to the likelihood of the null hypothesis given above. A likelihood ratio test can then be performed by using the statistic
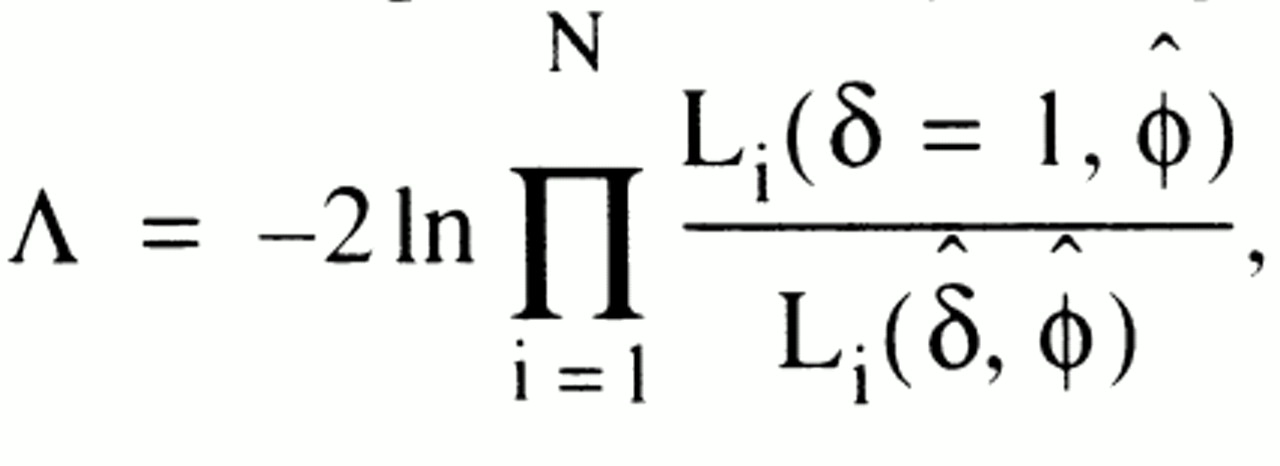
where ϕ and δ are estimated over all sibships jointly. This likelihood-based test follows a chi-square distribution, and it was considered to be one-tailed (with p≤0.05 indicating significance), since the core hypothesis on increased concordance for sex under paternal transmission makes a definite prediction about the direction of the effect
RESULTS
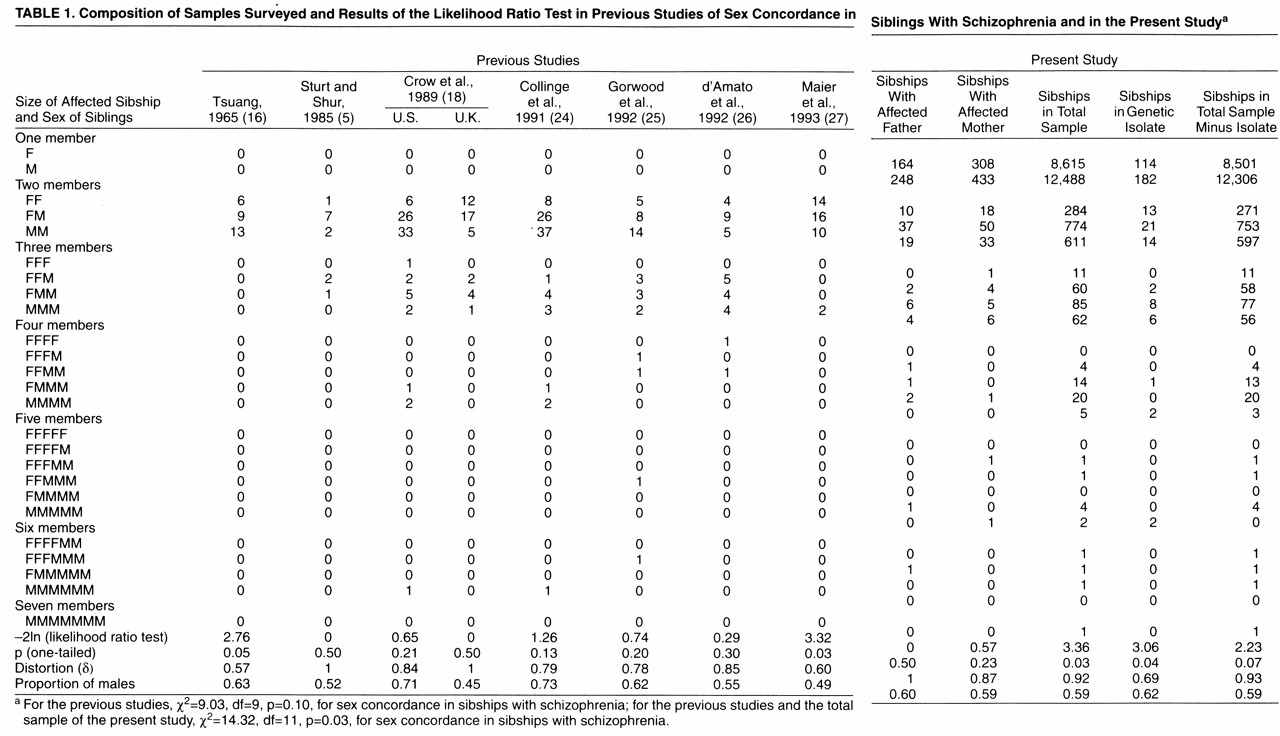
Of the 25,332 cases of schizophrenia (male-to-female ratio=1.46) in which the families could be identified, 3,338 were in sibships with two affected individuals, and a further 891 belonged to sibships with three or more affected members; there was a maximum of six affected individuals in three families and seven affected siblings in one instance (
table 1). By simulation of at least 200 replicates of that data set, we estimated that any distortion of sex concordance below 0.9, a deviance milder than those found in previous work (δ in
table 1), would essentially be identified with complete power (
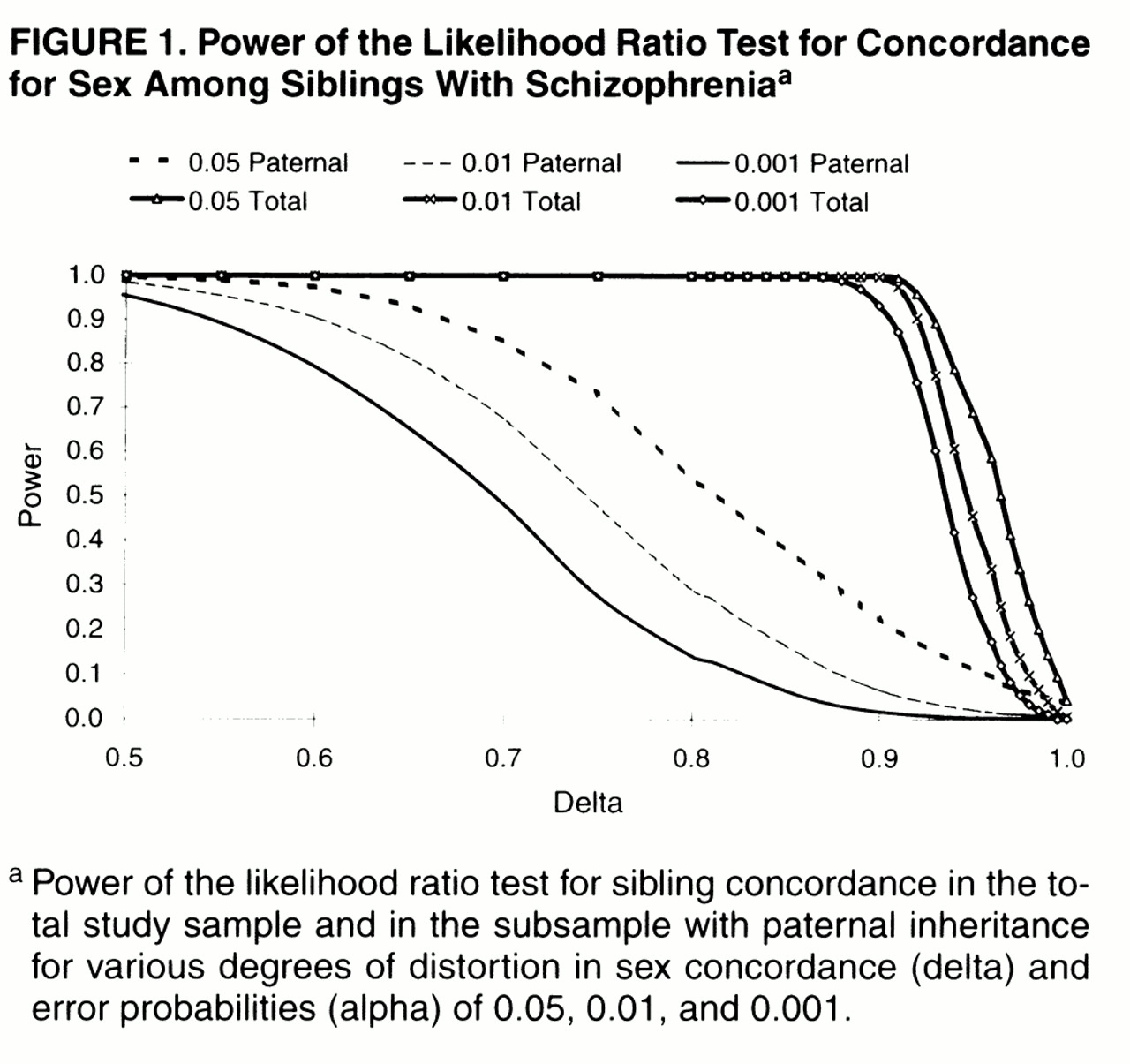
figure 1, curves for total sample).
Paternal Transmission
In eight of the affected sibling pairs and an additional triplet, both parents had a diagnosis of schizophrenia; these families were therefore excluded from the analyses by parental affection status. Only the fathers were affected in a further 86 sibships with multiple cases of schizophrenia, yet in two instances insufficient data on the mother were available, so we restricted the analysis to the 84 multiply affected sibships whose mothers proved to be not registered cases. However, there was no significant deviation from a random distribution of the sexes among affected siblings in this subset of families (χ
2=0, df=1, p=0.50), and considering all 86 sibships would have in no way changed the result. To exclude the possibility that this null finding had arisen out of the insufficient power of our subsample, we simulated at least 1,000 replicates and found that given the effect size of previous work, where raw data on sibships with paternal transmission are available (0.52<δ<0.55, calculated from reference
18), a distortion in sex concordance of that degree would have been identified more than 90% of the time, even under a conservative error rate of 0.1% (
figure 1, curves for paternal transmission).
Full Sample
Since the core hypothesis of increased sex concordance of affected sibling pairs under paternal transmission could not be confirmed, it might be expected that the full sample of 1,942 multiply affected sibships ascertained irrespective of parents’ diagnostic status would not show any significant deviation of sex concordance either. Surprisingly, we found mild evidence of slightly increased sex concordance in the nationwide sample (δ=0.92, χ
2=3.36, df=1, p=0.03). To test how much this unexpected finding depended on the impact of a particular isolated community in northeastern Finland that has been targeted for molecular genetic studies because of a twofold greater prevalence of schizophrenia, a larger family size, and a population history suggestive of an internal genetic isolate
(28), we excluded all cases from that community (166/4,229=3.9% of cases from sibships with two or more affected members;
table 1) and were left with no further evidence of increased sex concordance for the remaining larger part of Finland (δ=0.93, χ
2=2.23, df=1, p=0.07), whereas the internal genetic isolate alone would show some (δ=0.69, χ
2=3.06, df=1, p=0.04).
Maternal Transmission
In a subsample of 155 multiply affected sibships, the mothers but not the fathers were affected. However, since a Finnish mother is not obliged to reveal the identity of her newborn child’s father to the registration authorities, the fathers’ diagnostic data could not be traced sufficiently to exclude biparental transmission in several sibships. We therefore felt that it was safer to restrict this analysis to the 120 multiply affected sibships in whom schizophrenia could be definitely excluded by cross-checking with the case registers of their fathers. Again, the sex distribution of affected siblings did not differ from expectations under the null hypothesis (χ2=0.57, df=1, p=0.23), independent of whether sibships with unclarified paternal affection status were excluded or not.
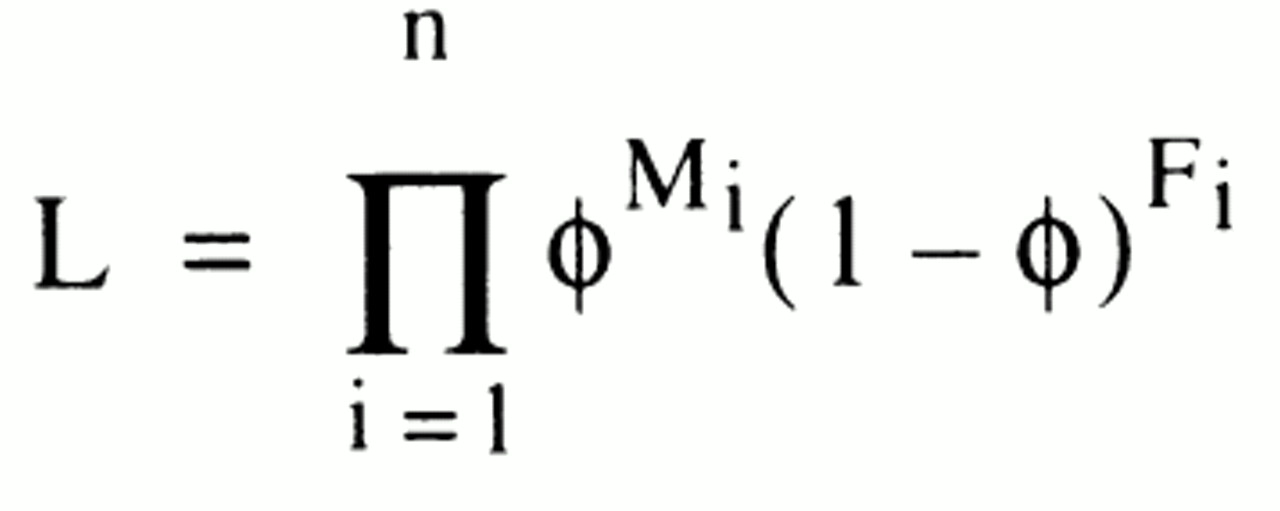 where ϕ is the probability of a random affected person being male, and the product is taken over all sibships, i. The maximum likelihood estimate of ϕ can be shown to be the binomial proportion
where ϕ is the probability of a random affected person being male, and the product is taken over all sibships, i. The maximum likelihood estimate of ϕ can be shown to be the binomial proportion 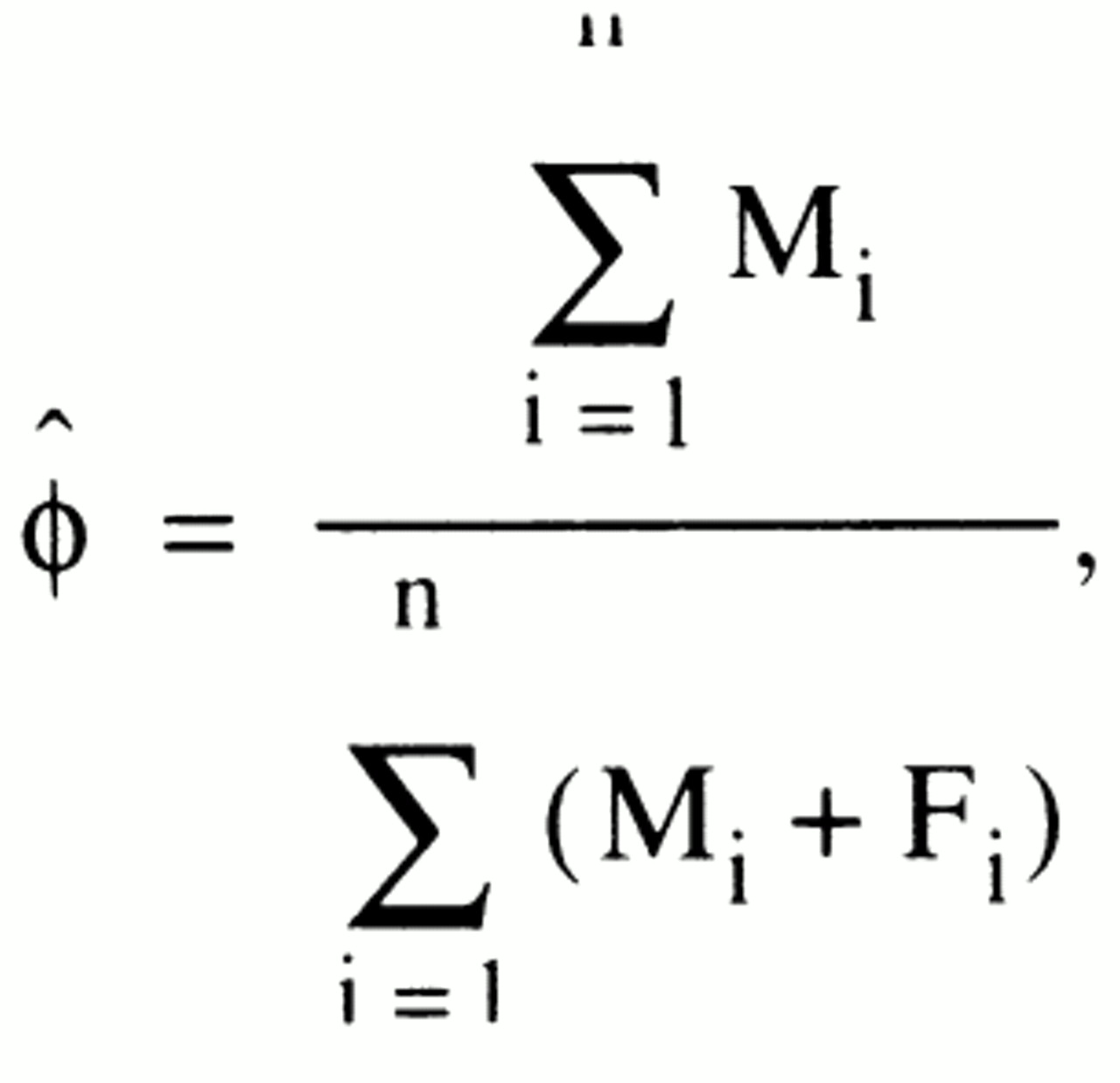 where Mi is the number of males in sibship i, and Fi is the number of females in the same sibship i. Under the alternative hypothesis, it is presumed that when the proband is male, there should be a reduced probability that remaining siblings are female, and thus the likelihood for a given sibship with a male proband is given as Li,male=ϕ[δ(1–ϕ)]Fi [1–δ(1–ϕ)]Mi–1, and with a female proband the likelihood would correspondingly be Li,female=(1–ϕ)[δϕ]Mi [1–δϕ]Fi–1. However, since the proband is typically unknown, one can determine the probability that a given sibship had a male proband as ψ=Mi / (Mi+Fi). Thus, the overall likelihood for a given sibship with an unknown proband would be Li=ψLi,male+(1–ψ)Li,female, and when δ=1, this is identical to the likelihood of the null hypothesis given above. A likelihood ratio test can then be performed by using the statistic
where Mi is the number of males in sibship i, and Fi is the number of females in the same sibship i. Under the alternative hypothesis, it is presumed that when the proband is male, there should be a reduced probability that remaining siblings are female, and thus the likelihood for a given sibship with a male proband is given as Li,male=ϕ[δ(1–ϕ)]Fi [1–δ(1–ϕ)]Mi–1, and with a female proband the likelihood would correspondingly be Li,female=(1–ϕ)[δϕ]Mi [1–δϕ]Fi–1. However, since the proband is typically unknown, one can determine the probability that a given sibship had a male proband as ψ=Mi / (Mi+Fi). Thus, the overall likelihood for a given sibship with an unknown proband would be Li=ψLi,male+(1–ψ)Li,female, and when δ=1, this is identical to the likelihood of the null hypothesis given above. A likelihood ratio test can then be performed by using the statistic 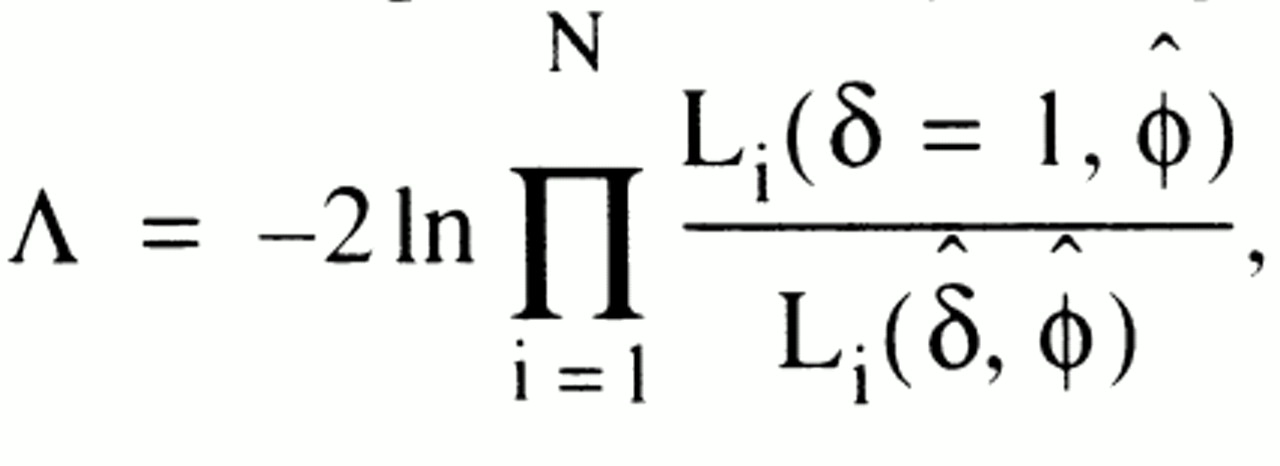 where ϕ and δ are estimated over all sibships jointly. This likelihood-based test follows a chi-square distribution, and it was considered to be one-tailed (with p≤0.05 indicating significance), since the core hypothesis on increased concordance for sex under paternal transmission makes a definite prediction about the direction of the effect
where ϕ and δ are estimated over all sibships jointly. This likelihood-based test follows a chi-square distribution, and it was considered to be one-tailed (with p≤0.05 indicating significance), since the core hypothesis on increased concordance for sex under paternal transmission makes a definite prediction about the direction of the effect