To illustrate the clinical characteristics of PANDAS, we present the case of a child with no prior psychiatric history who had a sudden and dramatic onset of tics and OCD following a GABHS throat infection.
CASE PRESENTATION
History of Present Illness
Frances was a 5-year-old Caucasian girl who presented to the outpatient psychiatric clinic at the National Institute of Mental Health (NIMH) for evaluation and treatment of sudden-onset motor and vocal tics and obsessive-compulsive symptoms.
Two months before her evaluation at NIMH, Frances had developed a hypersensitivity to her clothing. She began to complain that her socks and tights didn’t “feel right,” and she would change them several times until she found a pair that felt more comfortable. Over the following month, this hypersensitivity progressed to involve all clothing items. Every morning, she would change her clothing five times or more until it “felt right,” and Frances insisted that her mother cut off all the tags from her shirts, so that they would feel “more comfortable.”
A few weeks later, Frances had an abrupt onset of both motor and vocal tics. Her parents recall the exact day when her tics began. The eye-rolling tic started first, and within 24 hours, she had nearly constant eye-blinking, head-jerking, and nose-rubbing tics. Frances’s parents were so concerned about this sudden change that they took her to the local hospital’s emergency department. Assessment included a physical examination and CBC, both with normal results, as well as a computerized tomography scan of the brain with negative results No throat culture was done. According to the child’s mother, the emergency room doctors did not give a diagnosis because “they weren’t really sure what was wrong”; consequently, they prescribed no therapy.
Several days later, Frances had an equally abrupt onset of obsessions and compulsions, involving ordering and arranging, contamination, counting, and hoarding. Her parents noticed that she suddenly had to have all her crayons in a certain order or she would become quite distressed. She became so preoccupied and concerned about the order of her crayons that she no longer enjoyed coloring, which had been one of her favorite activities. When she did use her crayons, she would place a pencil in the spot where she removed the crayon so she could replace it in the correct position. About the same time, Frances became fearful of insects. This was particularly noticeable because she had always been fascinated by insects, even letting them crawl up her arm. She became excessively concerned about sitting on insects, fearing she might kill them, and she took extensive measures to avoid them. She also developed contamination worries, particularly involving urine and feces. Whenever she had to use the toilet, Frances would cover her mouth and blow out air to avoid inhaling the germs. She told her mother, “I’m blowing out the air because my poopy is dirty and I don’t want it to go into my mouth.” However, she did this even when she urinated. She would also spit if she saw anything that she considered “dirty,” like garbage cans.
Frances also developed compulsive counting and hoarding, and she began to save uneaten food, trash, and food wrappers. She would turn in circles and count to four; in addition, she would compulsively rub and touch objects “until it felt right.” Furthermore, she developed an elaborate mealtime ritual. She had to pray before every meal, and each prayer had to end with “I love you, Lord.” Everyone in the family had to remain silent while she whispered the prayer to herself, and no one could begin eating until she had finished her prayer. If she was interrupted, Frances would become upset and would have to say the prayer over again from the beginning. After completing the prayer, she would line up several stuffed animals and feed each one a bite of food before taking a bite herself. Because of this ritual, meals took twice as long as usual. If her parents attempted to interrupt her eating ritual, she would become very upset and would refuse to eat (this happened frequently enough that her weight decreased from 47 to 45 pounds over a 1-month period).
Around the same time that her tics and OCD began, Frances suddenly developed separation anxiety, insomnia, nightmares, mood lability, clumsiness, decreased concentration, and restlessness. Of these, the separation anxiety was most notable. Because Frances had worries that she or her mother would die or that she would be kidnapped, she refused to go into rooms alone and demanded that her mother or father stay with her at night until she fell asleep. When her mother had to leave the house, Frances would hang onto her mother’s leg and beg her not to go, behaving like a much younger child. In addition to the increased clinginess, Frances became much more irritable. She had frequent temper tantrums, and minor incidents would trigger extreme emotional reactions.
During this period, Frances also became restless and fidgety. She was unable to sit through a meal and her mother related that “she never stopped having movements.” Further, her drawing and painting appeared less neat, and she scribbled more. Moreover, her concentration and attention span decreased, and she was unable to complete projects that she used to finish easily. She developed sudden clumsiness, as well; her running appeared uncoordinated, and her jumping and climbing were “jerky.”
Recent Past Medical History
Frances had been ill with recurrent fevers for 3 months before the onset of the tics and OCD (she spiked a fever every 3 to 4 weeks for 3 months). She was examined by several physicians during this period, and it was presumed that she had a chronic viral infection. A chest X-ray was done, and the results were normal; however, a throat culture was not done until her mother requested it several weeks after the onset of Frances’s tics (when her mother learned of the possible association between GABHS and tics and OCD). At that time, Frances had no complaints of pharyngitis, but her throat culture was positive for GABHS. She was treated with a 10-day course of amoxicillin (250 mg orally t.i.d.). Within several days of starting the amoxicillin, Frances’s tics markedly decreased and almost disappeared, although her OCD did not improve. However, within 2 days after the amoxicillin was discontinued, she developed a fever (temperature to 104˚C), and the tics returned with greater complexity, intensity, and frequency. At this time, her tics included, among others, repetitive eye blinking, head jerking, jumping, shoulder shrugging, and multiple vocal tics (screaming, throat clearing, coughing, and noisy breathing), as well as blocking (stopping in midsentence). Because of her fever, the amoxicillin treatment was restarted at 125 mg b.i.d., although another throat culture was not taken. Frances remained on this dose of amoxicillin until she was evaluated in NIMH outpatient psychiatry clinic. Within a day or two of resumption of the amoxicillin, the tics again markedly decreased but did not remit, and her OCD continued to progress. At the time of her initial evaluation, her obsessions and compulsions consumed several hours a day with a total score of 24 (range=0–40) on the Children’s Yale-Brown Obsessive Compulsive Scale (7, 8). At that time, her tics were infrequent with a total score (combined motor and vocal tic scores) of 13 (range=0–50) on the Tourette Syndrome Unified Rating Scale
(9).
Past Psychiatric History
Frances had no previous history of emotional or behavioral problems, and she had not received any psychiatric evaluations or treatment before her initial assessment at NIMH. She also had no history of sexual or physical abuse or neglect.
Past Medical History
During her infancy and early childhood, Frances had had recurrent otitis media and sinusitis (often untreated), which did not require prophylactic antibiotics or myringotomy tubes. Before the present positive throat culture, she had no known streptococcal throat infections nor had she had scarlet fever, rheumatic fever (including Sydenham’s chorea), or an autoimmune disorder.
Social/Developmental History
Frances’s mother had taken clomiphene citrate before conception, and progesterone was prescribed throughout the pregnancy because of a difficult previous pregnancy. Frances, born full-term by normal spontaneous vaginal delivery, weighed 6 pounds, 13 ounces. Her Apgar scores were 9 and 10. She did not require special care and was discharged after 2 days.
Her development had been normal. She achieved developmental milestones at the appropriate times, except for a mild phonological disorder for which she received speech therapy.
At the time of her initial evaluation, Frances had completed her first year of preschool, and was to start kindergarten in the fall. She had been a delightful preschooler who was described by her preschool teacher as “easy to get along with . . . kids really like her . . . always has a positive attitude.” During her preschool years, Frances had no problems with her attention span, she was not impulsive or hyperactive, and she was well coordinated.
Frances had always lived with her mother, father, and older brother in a suburban upper-middle-class neighborhood. Both her parents had graduate degrees. Her mother worked part-time as a child psychologist, and her father was an engineer. Her parents were happily married. They were attentive, loving, and highly supportive of Frances.
Family History
Frances is the second of two children born to her mother and father. Her older brother was healthy but had mildly delayed fine motor skills. In her early 20s, Frances’s mother had experienced transient trichotillomania, which she reported was associated with a stressful life event and lasted less than a month; she also had a history of several episodes of mild depression that responded to psychotherapy. Frances’s maternal grandmother had a history of trichotillomania. Frances’s father had a history of vocal tics. There is no known family history of OCD or rheumatic fever, including Sydenham’s chorea.
Mental Status Examination
At the time of her initial evaluation, Frances was an attractive girl who appeared her stated age. She was friendly and cooperative, and she maintained good eye contact with the examiner. She had no difficulty separating from her parents to meet with the examiner. Throughout the session, she was restless and fidgety, but she was able to remain seated in her chair. She had frequent and obvious eye-blinking tics. Her speech was spontaneous at a regular rate, rhythm, and intensity with mild articulation difficulties. Her mood was anxious with a full affect. There was no evidence of psychosis, and she denied suicidal and homicidal ideation. She was alert and fully oriented. Her insight and judgment appeared to be age appropriate.
Treatment and Clinical Course
Frances met DSM-IV criteria for both OCD and transient tic disorder. In addition, she fulfilled the criteria established for PANDAS
(6).
At the time of her initial evaluation, laboratory studies revealed elevated antistreptococcal antibody titers (antistreptolysin O and antistreptococcal deoxyribonuclease-B) and a throat culture that was negative for GABHS. She also had an electroencephalogram, electrocardiogram, and brain magnetic resonance imaging (MRI), all with normal results.
Frances was enrolled in a randomized placebo-controlled trial of plasma exchange and intravenous immunoglobulin for PANDAS. She was admitted to the pediatric medical unit at NIMH and was randomized to receive intravenous immunoglobulin or sham intravenous immunoglobulin (placebo). She received 1 g/Kg of intravenous immunoglobulin or placebo per day for 2 consecutive days. She tolerated the infusion well and had no serious adverse effects, although she vomited once and had some mild nausea and a low-grade fever during the infusion. Following the completion of the blinded infusion, Frances was discharged on a regimen of prophylactic doses of amoxicillin (250 mg orally b.i.d.).
Two weeks after treatment with intravenous immunoglobulin or placebo, Frances was evaluated in the clinic for follow-up ratings. Her tics were almost gone, occurring less than once a day (Tourette Syndrome Unified Rating Scale score=4), and her obsessive-compulsive symptoms were reduced to a subclinical level (Children’s Yale-Brown Obsessive Compulsive Scale score=10). Her separation worries had disappeared, and she was much less anxious. She also had fewer nightmares and less emotional lability.
Frances continued to improve over the next 2 weeks; by 1 month after treatment, her OCD had almost remitted, except for minimal compulsive behaviors (Children’s Yale-Brown Obsessive Compulsive Scale score=3). She was no longer fidgety or restless, and her tics continued to be minimal and infrequent (Tourette Syndrome Unified Rating Scale score=4). Her mood was also back to baseline—she was less emotionally labile and no longer experienced sustained irritability. At this time, the “blind” was broken and it was revealed that she had received active treatment with intravenous immunoglobulin.
By 2 months after treatment with intravenous immunoglobulin, Frances’s OCD had completely remitted (Children’s Yale-Brown Obsessive Compulsive Scale score=0), and her tics continued to be mild (Tourette Syndrome Unified Rating Scale score=6); the decision was made that she did not require psychotropic medications or behavioral therapy.
Frances was completely free of obsessive-compulsive symptoms until 3 months after treatment when she developed an upper respiratory infection. A throat culture was negative for GABHS. Her antistreptococcal deoxyribonuclease-B titer, however, increased from 480 to 680. At that time, her tics, emotional lability, separation anxiety, and contamination obsessions suddenly returned. Her obsessive-compulsive symptoms were 30% to 50% as severe as they had been before treatment (Children’s Yale-Brown Obsessive Compulsive Scale score=12), but her tics were more severe than they had been at baseline (Tourette Syndrome Unified Rating Scale score=18). For the first time, Frances experienced complex tics, such as spinning (“I feel like there is a rope around me and . . . I spin until it feels right”). This exacerbation continued for several weeks until the amoxicillin was increased from prophylactic doses to treatment doses (250 mg t.i.d.). By 4 months after intravenous immunoglobulin, Frances’s tics were once again within tolerable limits (Tourette Syndrome Unified Rating Scale score=9) and her obsessive-compulsive symptoms had disappeared (Children’s Yale-Brown Obsessive Compulsive Scale score=0).
Frances continued to do well until 5 months after intravenous immunoglobulin when she developed an acute illness with sore throat, nausea, and headache. This was followed by an exacerbation of her neuropsychiatric symptoms. Her amoxicillin dose was increased to 250 mg t.i.d. for 10 days, but this time there was no associated improvement. Her symptoms remitted over the next 2 weeks; at her 6-month follow-up, Frances’s OCD was in remission (Children’s Yale-Brown Obsessive Compulsive Scale score=0), and tics were the same as pretreatment level (Tourette Syndrome Unified Rating Scale score=14).
Frances’s OCD remained in remission, and her tics were mild until 10 months after intravenous immunoglobulin when she once again developed pharyngitis. Her tics suddenly increased, and she again developed emotional lability, sensory hypersensitivity, separation anxiety, and increased OCD symptoms. One month later (11 months after intravenous immunoglobulin), she was evaluated in the clinic, and it was noted that her Children’s Yale-Brown Obsessive Compulsive Scale score had increased to 17 and her Tourette Syndrome Unified Rating Scale score, to 20. Although a throat culture was negative for GABHS, her streptococcal titers had increased significantly. She was once again treated with amoxicillin (250 mg t.i.d.) to see if this would ameliorate her symptoms. However, there was no improvement in her symptoms. Three weeks later (12 months after intravenous immunoglobulin), because of Frances’s lack of response to the amoxicillin and the continued progression of her neuropsychiatric symptoms, it was decided to admit her for retreatment with intravenous immunoglobulin. At the time of retreatment, her Children’s Yale-Brown Obsessive Compulsive Scale score was 20, and her Tourette Syndrome Unified Rating Scale tic score was 21. Frances tolerated the 2-day infusion of intravenous immunoglobulin well and was discharged with continued symptoms.
One month following retreatment with intravenous immunoglobulin, Frances’s tics were 90% improved (Tourette Syndrome Unified Rating Scale score=6), and her obsessive-compulsive symptoms were 70% improved (Children’s Yale-Brown Obsessive Compulsive Scale score=10).
DISCUSSION
Dr. Swedo, how do you determine if a patient has PANDAS?
Dr. Swedo: PANDAS is not a diagnostic label, but an acronym designating a subgroup of children with OCD and tic disorders whose symptoms appear to be triggered by streptococcal infections. As we have learned more about the clinical characteristics of the PANDAS subgroup, the inclusionary criteria have evolved, and they may change further as we learn more about the salient features of the population
(3, 5). For example, although a diagnosis of OCD or a tic disorder is required for inclusion in the PANDAS subgroup, other neuropsychiatric symptoms—emotional lability, separation anxiety, anorexia, as well as impulsivity, distractibility and motoric hyperactivity reminiscent of attention deficit hyperactivity disorder (ADHD)—also occur frequently
(6, 10). It is possible that these primary diagnoses may eventually be included in the PANDAS criteria.
The fourth criterion, temporal association between GABHS infections and symptom exacerbations, is the most important one for determining whether or not a child should be included in the PANDAS subgroup. The relationship between symptom exacerbations and GABHS infections must be established by demonstrating that the symptoms worsen in association with streptococcal pharyngitis and remit when the child is free of GABHS. Ideally, this is done prospectively by identifying the presence of a GABHS infection during at least two neuropsychiatric relapses. The decision, however, is usually made retrospectively by comparing the child’s psychiatric history with her pediatric medical records to determine whether or not GABHS infections result in symptom exacerbations. To ensure that the association is real and not a sampling error, during a relapse, the child should have a positive throat culture accompanied by rising antistreptococcal titers (two-fold rise in month following infection), or elevated titers and a recent history of pharyngitis. The mere presence of elevated titers is not sufficient proof of a recent GABHS infection because antistreptococcal titers, such as the antistreptolysin O or antistreptococcal deoxyribonuclease-B, can remain elevated for several months following a GABHS infection. Similarly, a positive throat culture is not sufficient proof of a current streptococcal infection because of the possibility of GABHS carriage in the nasopharynx (the carrier state is defined by a lack of immune response to GABHS, i.e., a positive throat culture without accompanying titer rise). Proving an association between neuropsychiatric symptom exacerbations and GABHS infections is further complicated by the fact that children with PANDAS can have symptom relapses in the absence of a preceding GABHS infection (either in association with a non-GABHS infection or spontaneously, as occurred in Frances’s case). During a period of neuropsychiatric symptom remission, however, a child with PANDAS should not have an active GABHS infection or rising antistreptococcal titers—thus making it as important to draw titers during remissions as during relapses. Because antistreptococcal titers are expensive and the results do not yet influence the patient’s treatment regimen, they are not warranted currently in the routine clinical care of children with OCD or tic disorders.
Dr. Perlmutter, why was this case chosen as illustrating the clinical course of PANDAS?
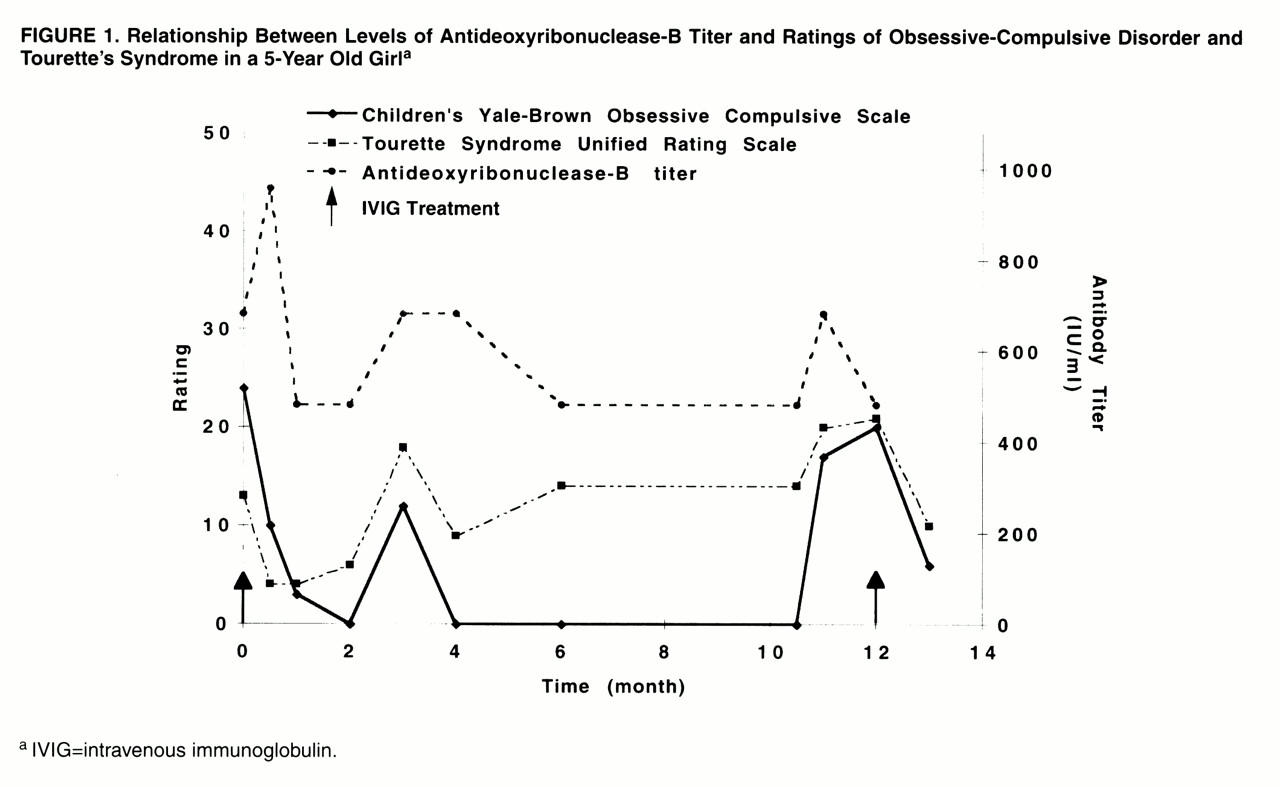
Dr. Perlmutter: As seen in
figure 1, Frances’s tics and obsessive-compulsive symptoms had an episodic (relapsing-remitting) pattern—there were periods of time when her symptoms were acutely and dramatically worse, and periods of time when her symptoms were in complete or partial remission. The antistreptococcal deoxyribonuclease-B titer followed the same pattern as her clinical course—it was elevated during times of symptom exacerbations and decreased during periods of partial or complete remission. All of her symptoms rapidly improved (within 2 weeks) after treatment with intravenous immunoglobulin and remained stable and under control until she became ill and her antistreptococcal deoxyribonuclease-B titer elevated.
The acute onset of symptoms (within 24–48 hours) and the episodic relapsing-remitting clinical course are distinguishing features of PANDAS and are in contrast to the more typical waxing-waning symptom pattern often seen in children with OCD and tic disorders
(6, 9).Dr. Rapoport, how does Frances’s clinical course differ from classic childhood OCD (non-PANDAS)?
Dr. Rapoport: This question is at the moment unanswerable but very important. We have observed for a long time that children with OCD or Tourette’s disorder who are maintained on a regimen of “ideal” antiobsessional or tic medication still can have sudden and dramatic changes in symptom severity. No one had looked systematically into the relation to streptococcal or other infections before. Earlier in our project, however, a staff fellow, struck by infections in pets occurring in some children in the weeks before their symptom exacerbations, was pursuing this avenue of research. In short, PANDAS may be in fact a more representative group of childhood OCD than we realize, but the facts are not in.
Dr. Perlmutter, please discuss clinical symptoms of PANDAS and how it is illustrated in this case.
Dr. Perlmutter: We have found that the mean age at onset of OCD or tic disorder in children who have PANDAS (6.3 years for tics and 7.4 years for obsessive-compulsive symptoms) is almost 3 years younger than for previously reported samples of childhood-onset OCD and tic disorders
(6). Frances was 5 years old when she suddenly developed tics and OCD. In addition, she presented with comorbid behavioral changes that were clearly distinguishable from her premorbid state: sudden onset of separation anxiety, irritability and emotional lability, nightmares, clumsiness, motoric hyperactivity and fidgetiness, and age regression. These behavioral symptoms were episodic and temporally related to her GABHS infections; they followed the same clinical course as her OCD and tic symptoms. This pattern is similar to that seen in the majority of patients with PANDAS
(6).
Dr. Castellanos, what is the relationship of ADHD to PANDAS? Should the age requirement (of having impairing symptoms before the age of 7 years) rule out the diagnosis of ADHD?
Dr. Castellanos: The diagnosis of ADHD remains a syndromic one, akin to fever. While the majority of cases of ADHD are related primarily to genetic susceptibility, there are a wide range of other possible causes, including, no doubt, autoimmune dysfunction in the CNS. The symptoms of ADHD appear to reflect dysfunction of the brain circuits that underlie the performance of executive function, particularly the function of inhibition in the cognitive domain. The circuit through which these functions operate includes the prefrontal cortex, basal ganglia, thalamus, and cerebellum
(11). Most of these brain regions are organized in a regional manner, and it is likely that tics reflect dysfunction in a particular set of sensorimotor circuits, while obsessions and compulsions reflect similar dysfunctions in the limbic-related circuits
(12).
The criterion that impairing symptoms of ADHD must be present before the age of 7 years helps to exclude other disorders that also produce impairment of cognitive function, such as mood, anxiety, or psychotic disorders, which typically have later times of onset. However, if a child had a sudden onset of ADHD symptoms after the age of 7, the diagnosis could still be ADHD but would be qualified as not otherwise specified (314.9).
Dr. Perlmutter, what was Frances like premorbidly (before the onset of PANDAS)?
Dr. Perlmutter: She had none of the premorbid characteristics that would predict the later development of an anxiety or mood disorder. She did not have a history of behavioral inhibition, obsessive-compulsive behaviors, separation anxiety, oppositionality, or sustained periods of irritability or depression. She was a well-adjusted preschooler with age-appropriate social skills.
Dr. Castellanos, please address the sensory defensiveness and hypersensitivity exhibited by this patient and the progression to tics and OCD.
Dr. Castellanos: This is the type of clinical clue that supports the notion that these three disorders all relate to dysfunction of circuits that include the basal ganglia. The caudate and putamen (also referred to as the striatum) are histologically indistinguishable, but there are multiple levels of organization in these structures that have functional significance. Thus, the dorsolateral striatum is associated primarily with sensorimotor function, and lateral regions of the striatum are most closely linked to cognitive processing, whereas limbic cortical regions are connected to ventral striatum, and especially to the nucleus accumbens
(12). We do not yet understand the particular differences between these zones, but it is clear that certain neuroleptics have regionally specific effects. For example, clozapine produces robust activation of the c-fos system in limbic regions of striatum and accumbens, while the “inverse neuroleptic,” metoclopramide, produces a complementary pattern of activation that is relatively restricted to sensorimotor regions
(13). It is possible that autoimmune dysfunction can cause regions within the basal ganglia to be less able to provide their usual inhibitory output, resulting in a diminished ability to gate or filter out sensory stimuli, for example. This would account for the sensory hypersensitivity and defensiveness. It is now believed that tics in many cases are primarily triggered by abnormal sensations
(14), thus, Frances’s tics could have followed from deficient sensory gating, with the tics being underinhibited movements. Likewise, deficient inhibition of normal anxieties or worries may have resulted in Frances’s new onset of separation anxiety and OCD.
Dr. Giedd, should brain MRIs be a routine part of the workup for PANDAS?
Dr. Giedd: Because of the large variability of normal basal ganglia sizes, the high cost, and the poor sensitivity and specificity of findings, acquisition of an MRI scan is not warranted in the evaluation of PANDAS. This is generally the case for other child psychiatric disorders as well. Although group differences in brain anatomy have been found for autism, ADHD, childhood onset schizophrenia, dyslexia, fetal alcohol syndrome, learning disabilities, OCD, posttraumatic stress disorder, Sydenham’s chorea, and Tourette’s disorder
(15), there is currently no specific brain MRI finding common to all, or even most, children with any of these disorders. Clinical indications for an MRI scan include movement disorders of uncertain etiology, anorexia nervosa (to rule out pituitary tumors), first episode of psychosis, or severe mood or psychotic disorders not responding to conventional treatments. For the remainder of the disorders, MRI remains primarily a research tool to help elucidate the core pathophysiology and neurobiology of illnesses.
Dr. Garvey, please comment on the correlation between Frances’s streptococcal titers and her neuropsychiatric symptoms (OCD and tics).
Dr. Garvey: Rising (or elevated) streptococcal titers indicate that a GABHS infection has occurred. Both antistreptolysin O and antistreptococcal deoxyribonuclease-B titers are accurate and valid; however, some infections may stimulate only one of these antibodies. Thus, if the clinical suspicion for a GABHS infection is high, it may be worth doing both tests or, perhaps, repeating the titer 2 to 3 weeks later to see if there has been a change. A rise in these titers is the most specific evidence of a recent GABHS infection. Prospective studies of patients with streptococcal infections have shown that while the antistreptolysin O titer typically rises between 3 and 6 weeks after an infection, the antistreptococcal deoxyribonuclease-B titer tends to rise later, 6 to 8 weeks after an infection
(16). There are three different patterns of antibody response to a GABHS infection. The first is a rapid rise and fall of antibody. This may take place over a matter of weeks. More typically, patients will have a slower rise over 2 or 3 weeks and a gradual fall over several months. A number of children, however, will have a rise in antibody that persists long after the infection has cleared. Thus, one would not expect streptococcal titers and neuropsychiatric symptoms to mirror one another exactly. Penicillin treatment may alter the course described above, since prompt treatment of GABHS infections can suppress or blunt the expected rise in streptococcal antibodies
(17, 18).
Dr. Swedo, why would Frances’s symptoms decrease during treatment with amoxicillin?
Dr. Swedo: I wish we knew the answer to that question, as it must certainly be one of the keys to understanding the pathophysiology of PANDAS. Unfortunately, at present, we don’t know the mechanism by which the symptom improvement occurs, nor do we know which children (or which episodes, as in Frances’s case) will respond to amoxicillin treatment. It is possible that Frances’s remissions were a placebo response to her NIMH visit or occurred spontaneously, as would be expected in an episodic disorder. Frances’s amoxicillin-associated remission, however, is one of many such occurrences observed at NIMH and elsewhere. The first and most dramatic example was several years ago, when Dr. Henrietta Leonard and I noted that a 10-year-old girl’s OCD completely remitted following amoxicillin treatment for otitis media. Before the antibiotic treatment, her obsessive-compulsive symptoms had been so severe that she was being evaluated for inclusion in the plasmapheresis/intravenous immunoglobulin treatment trial. We had planned to admit her to the hospital after she completed the 10-day course of antibiotics, but on the third day of amoxicillin therapy, her mother called to report that her daughter was “cured.” A double-blind trial of amoxicillin and placebo (A-B-A-B crossover) revealed that the amoxicillin effect was replicable: the patient relapsed within 2 to 3 days of placebo substitution and remitted each time the amoxicillin was reinstated. A similar controlled trial is ongoing at NIMH and at Brown University (Dr. Leonard) to investigate this effect, as well as to examine the immunologic changes that accompany the amoxicillin response, so perhaps the question of why symptoms decrease during amoxicillin treatment will be answered in the near future.
Dr. Mittleman, how do intravenous immunoglobulin and plasmapheresis work, and why might these treatments be effective for acute exacerbations of PANDAS?
Dr. Mittleman: There are many potential mechanisms that may be implicated in the effectiveness of intravenous immunoglobulin and plasma exchange in PANDAS and other autoimmune disorders. Important immunomodulatory effects may be common to both treatments and may be responsible for their efficacy or, alternatively, different pathways may govern the beneficial effects of the two treatments.
Intravenous immunoglobulin is the pooled immunoglobulin fraction (IgG) from thousands of donors. As such, it represents the cumulative humoral immune response of multiple individuals and contains IgGs of all subclasses and many V-region specificities for both exogenous and self-antigens. The IgG in the pharmacological preparations has intact Fc regions, thereby permitting binding to cells bearing Fc receptors; circulating proteins including complement, host idiotypes, microbial products, and so forth. The effect of Fc-receptor engagement on the cell surface may result in intracellular signaling or receptor blockade. The effect of Fc binding of circulating proteins may modulate inflammatory signals
(19).
Plasma exchange or plasmapharesis involves the ex vivo separation of blood into two fractions: formed elements and soluble components. The formed elements are returned to the circulation while the soluble fraction is replaced with a protein solution having similar physical characteristics (tonicity, pH, viscosity, etc.) but lacking many natural factors. The removed portion contains antibodies, cytokines, complement components, hormones, serum binding proteins, and many other diverse molecules. Plasma exchange is performed serially at intervals of 1 or more days between treatments to permit the establishment of an equilibrium between the circulation and interstitial or tissue compartments. The net effect of the serial removal of plasma volume and its replacement with a “biologically inert” solution includes the removal of Igs, which then cannot initiate signaling through the B-cell receptor or Fc receptors, and the removal of cytokines and hormones, which then cannot exert their immunomodulatory and functional effects
(20).
Through the mechanisms mentioned and others, both intravenous immunoglobulin and plasma exchange can be immunomodulatory (altering signals to T cells and B cells and resulting in altered functional responses); may cause immune deviation (the switch of one type of immune response to another); or may influence the immune repertoire by blocking or engaging a specific, antigen-driven, immune response important in the pathogenesis of PANDAS.