With the identification of REM sleep and the development of polysomnography about four decades ago, investigators have tried to characterize the nature of the prominent sleep disturbances in schizophrenia. Although some discrepant findings have been obtained, a polysomnography profile of schizophrenia might include 1) significant disruption of sleep continuity, 2) shortened REM latency with relatively normal REM time and density, and 3) reduced slow-wave sleep
(1–5).
State and trait aspects of these EEG sleep abnormalities and effects of antipsychotic treatment have, however, not been clearly defined. While previous studies have noted a relatively consistent improvement in sleep continuity in schizophrenic patients during the course of antipsychotic treatment
(6–8), effects on sleep architecture and REM sleep measures are less clear. REM latency is generally observed to increase (move toward normal)
(6, 8), while slow-wave sleep is unaffected
(7, 8). In an earlier study of 14 schizophrenic patients
(9), we observed an increase in REM latency without any change in slow-wave sleep during neuroleptic treatment. To replicate and extend these findings, we studied a nonoverlapping group of an additional 14 schizophrenic patients, by conducting EEGsleep studies before and 3 weeks after starting antipsychotic treatment in these subjects.
METHOD
The group consisted of 14 inpatients (12 men, two women) who met the criteria for schizophrenia of both the Schedule for Affective Disorders and Schizophrenia/Research Diagnostic Criteria and DSM-III-R. The mean age was 27.7 years (range=18–45), and mean duration of illness was 8.8 years (range=0.8–24). Study subjects were medication free for a minimum of 2 weeks before the initial sleep EEG recordings. Three subjects were psychotropic naïve, and 11 subjects had discontinued treatment (range=3–26 weeks) and were hospitalized for a psychotic exacerbation. We excluded subjects with significant medical or neurologic disease, clinical evidence of a primary sleep disorder, or a history of drug or alcohol abuse in the previous 6 months. Written informed consent was obtained from study participants.
Patients underwent 2 nights of premedication sleep EEG monitoring. Daytime napping was not allowed on sleep study days. After 2 nights of pretreatment polysomnography were completed, neuroleptic therapy was begun with clinically determined doses of haloperidol (N=7) or thiothixene (N=7) (mean haloperidol equivalent=11.4 mg/day). Ten patients were treated for extrapyramidal symptoms with benztropine (mean dose=2.5 mg/day). After an average of 23 days of pharmacological treatment, 2 more nights of polysomnography were recorded. A complete description of the polysomnography methods is provided in earlier publications
(4, 9). The first night of recording allowed acclimatization of the subject; the second-night pretreatment polysomnography data were used in the analysis. Blind ratings of sleep records, which used modified Rechtschaffen-Kales criteria
(10), were derived.
The following sleep continuity measures were assessed: time spent asleep, sleep latency, number of arousals, time awake after sleep onset, sleep efficiency (time spent asleep divided by total recording period), and sleep maintenance (time spent asleep divided by total recording minus sleep latency). REM latency was defined as the time from sleep onset until the onset of the first REM period of 3 minutes or more in duration, minus intervening time awake. Other REM measures included REM activity (sum of visually scored eye movement density from each 6-second REM epoch, on a 0–8 scale), total REM time, and REM density (REM activity divided by REM time). Each REM measure was examined for the entire night and for the first REM period. Sleep architecture was designated as the amount of time spent in stages 1, 2, 3, and 4, taken as a percentage of time spent asleep. Delta percentage was the sum of stages 3 and 4, taken as a percentage of time spent asleep.
Two-tailed repeated measures Student’s t test to compare sleep measures at drug-free baseline and after 3–4 weeks of neuroleptic treatment were performed. Because of the skewed distributions of some of the polysomnography variables, appropriate data transformations to normalize the data (logarithmic or square root) were performed. Sleep latency, REM latency, and percent delta sleep were the primary measures compared, since these are known to be abnormal in schizophrenia. Additional comparisons of other sleep measures were conducted secondarily.
RESULTS
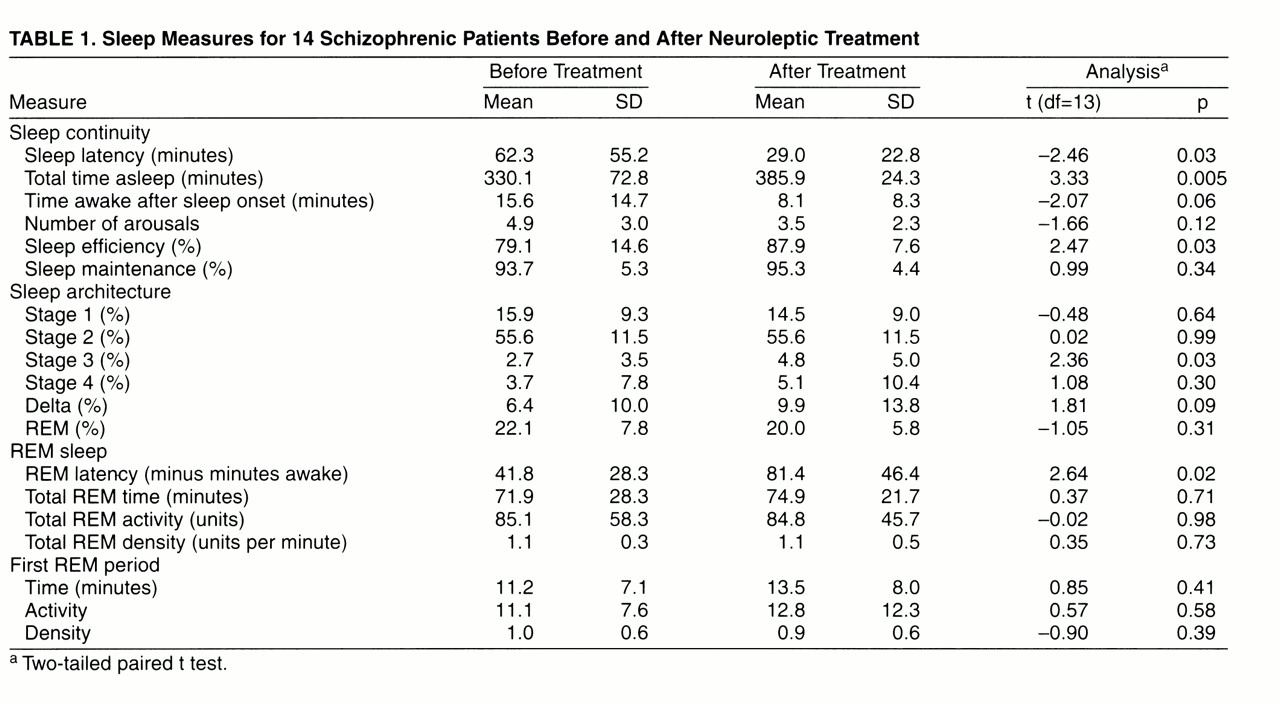
After 3–4 weeks of neuroleptic treatment, sleep continuity improved significantly (
table 1): 1) sleep latency decreased (treated patients fell asleep earlier), 2) total time asleep increased, and 3) sleep efficiency improved. Treated patients also showed less wakefulness, fewer arousals, and modestly improved sleep maintenance.
Following antipsychotic treatment, the only significant change in sleep architecture was an increase in stage 3 slow-wave sleep. There was a trend toward an increase in percent delta sleep. REM latency increased significantly after antipsychotic treatment, from an average of 42 minutes to a mean of 81 minutes. However, five of the 14 patients continued to exhibit intervening time awake of less than 60 minutes even after 3–4 weeks of antipsychotic treatment. There was no mean difference in REM latency or change in intervening time awake for the 10 people receiving an oral anticholinergic agent and for the four patients not receiving such an agent. Other REM measures did not differ significantly before and after treatment.
DISCUSSION
With neuroleptic treatment and clinical improvement over 3.5 weeks, the following polysomnography changes were noted: 1) sleep continuity improved (sleep latency decreased, time spent asleep increased, and sleep efficiency improved, 2) REM latency increased, and 3) stage 3 slow-wave sleep and total low-wave sleep increased. Although these sleep measures moved toward normalization with treatment, they continued to be abnormal as revealed by comparison to normative published data, as well as those from our laboratory
(4).
The open, uncontrolled nature of the study warrants cautious interpretation of its findings. The use of visual ratings of slow-wave sleep and the absence of computerized assessments limit interpretability of the slow-wave sleep findings.
This study essentially replicates findings from our previous study
(9) and is consistent with other reports
(6–8). All studies confirm improvement in sleep continuity in schizophrenic patients as their psychotic symptoms improve during neuroleptic treatment. A trend toward “normalization” of shortened REM latency in schizophrenic patients during the course of antipsychotic treatment is also noted consistently. As in previous studies
(8, 9), we observed no change in REM time or activity after 3–4 weeks of antipsychotic treatment. We observed no net increase in slow-wave sleep, although a specific increase in one component—stage 3 slow-wave sleep—was observed. This suggests that reduced slow-wave sleep may be a trait abnormality in schizophrenia, as previously suggested
(1, 8). It should be noted that shortened REM latency may be due in part to decreased slow-wave sleep
(1, 11).
The neurobiological basis of these state and trait EEG sleep abnormalities in schizophrenia is unclear. The state abnormalities (shortened REM latency and disrupted sleep continuity) can be explained by increased muscarinic cholinergic activity, whereas the trait abnormality (reduced slow-wave sleep) can be explained by early/late developmental structural brain abnormalities that are hypothesized in the illness
(4, 8, 9, 11, 12). Further studies are necessary to evaluate this possibility. Whether atypical and conventional antipsychotics differ in their effects on EEG sleep measures in schizophrenia is unclear; emerging data suggest some differences, particularly with regard to REM sleep
(13, 14). Polysomnography is a “window onto the brain,” and EEG sleep studies may provide useful information about the pathophysiology of schizophrenia and how this is affected by treatment with typical and atypical antipsychotic agents.