Childhood-onset schizophrenia is a topic of special interest. It is defined by the onset of psychotic symptoms by age 12 years and is quite rare, with recent estimates suggesting that it occurs at one-fiftieth the rate of the adult-onset form (
1). Unusually early onset of other multifactorial diseases, such as diabetes mellitus and rheumatoid arthritis, demonstrates greater heritability and disease severity (
2). The fact that such early onset of schizophrenia is rare has led to speculation that this form represents a particularly severe illness, and that the early onset reflects a stronger biological disposition (
3). If indeed the childhood-onset form has the same etiology and pathophysiology as the adult-onset form, it may well represent a more homogeneous, more severe form of the illness. Study of the early-onset population may then provide the opportunity to better discern the neural underpinnings—the brain pathology—of schizophrenia.
In the literature on adult patients, there is mounting evidence that a subset of patients have brain abnormalities which have probably been present from birth, indicating early abnormal brain development. Specific developmental brain anomalies associated with adult-onset schizophrenia include corpus callosum agenesis/dysgenesis (
4,
5) and gray matter heterotopia, a neuronal migration anomaly (
6). In addition, there have been several studies, including one recently published by our group (
7), of increased frequency of an enlarged cavum septi pellucidi (CSP) in patients with schizophrenia (
4,
8–
11). The rates of corpus callosum agenesis and gray matter heterotopia in schizophrenia are very low (1.9% [4] and 1.8% [12], respectively). However, several studies have shown that the frequency of enlarged CSP in adult-onset patients is quite high, ranging from 15% (
4) to 45% (
10). It has been speculated that an enlarged CSP represents a forme fruste of midline abnormalities, a marker for limbic system dysgenesis, or both (
13–
15). In addition, an enlarged CSP has been associated with cognitive deficit in developmental disorders such as mental retardation (
15) and Apert's syndrome (
16).
To date there have been no imaging studies examining the frequency of enlarged CSP in childhood-onset schizophrenia. Many aspects of the childhood-onset form of schizophrenia are quite similar to the adult-onset form but appear exaggerated; persons with the childhood-onset form have poorer premorbid functioning in a variety of areas, including delay in language development, speech and language disorders, learning disorders, motor stereotypies, and disruptive behavior disorders (
17). This suggests that the childhood-onset form may be a more severe developmental disorder than the adult-onset form. However, questions still remain: Do patients with the childhood-onset form of schizophrenia have septum pellucidum anomalies as seen in the adult-onset form? Do they occur at the same frequency? Are they more severe?
This study was a collaborative effort of the Child Psychiatry Branch of the National Institute of Mental Health (NIMH) and the Mental Health Clinical Research Center at the University of Iowa to investigate the frequency and severity of CSP enlargement in a group of patients with childhood-onset schizophrenia compared with healthy subjects.
METHOD
A total of 24 patients (12 male and 12 female) with childhood-onset schizophrenia were included in this study. The subjects were assessed and recruited at the Child Psychiatry Branch of NIMH for a double-blind inpatient trial of haloperidol and clozapine. The inclusion criteria were 1) age between 6 and 18 years, 2) a DSM-III-R diagnosis of schizophrenia with documented onset of psychotic symptoms by age 12, and 3) a full-scale IQ of 70 or above. In addition, as required for clozapine treatment, the patients had to have had at least two previous unsuccessful neuroleptic trials. This study group represents the addition of three patients to the group of 21 used in a previous study on regional brain morphology (
18).
Inclusion in the study was based on a full day of screening, which included review of old records, clinical interviews with parents and child, and structured interviews incorporating sections of the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (
19) and the Diagnostic Interview for Children and Adolescents, Revised Version (
20). After complete description of the study, written informed consent was obtained from the subjects' parents, and the subjects gave assent.
The mean age at admission to the study for the group of patients was 14.6 years (SD=2.1, range=9–19), the mean age at onset of psychosis was 10.2 years (SD=1.8, range=5–13), the mean length of prior hospitalization was 9.5 months (SD=13.5, range=0–53), and the mean length of treatment with neuroleptics was 22.9 months (SD=15.8, range=2–54). Twenty-five percent of the patients were left-handed, 5% showed mixed handedness, and 70% were right-handed. Parental socioeconomic status was determined with the use of a modified Hollingshead scale (
21). This scale ranges from 1 to 5; the lower the number, the higher the social class. The mean parental socioeconomic status for the patient group was 2.96 (SD=1.06).
Healthy comparison subjects were also recruited by the Child Psychiatry Branch at NIMH. A total of 95 comparison subjects (44 female and 51 male) were included in this study. Their mean age was 11.7 years (SD=3.4, range=4.7–17.8). They were recruited from the community through advertisements and screened by means of telephone interviews and parent/teacher rating scales. Potential subjects were then assessed in person with physical and neurological examinations, a structured psychiatric interview that used the child and parent versions of the Diagnostic Interview for Children and Adolescents (
22), the Child Behavior Checklist (
23), the vocabulary, block design, and digit span subtests of the WISC-R, and the Conners parent and teacher questionnaires (
24,
25). Potential comparison subjects were excluded if there was any physical or neurological disorder, a lifetime history of any psychiatric disorder, or a history of a major psychiatric disorder in first-degree relatives. Further details of the healthy subject recruitment and screening protocols have been published elsewhere (
26). The mean socioeconomic status for parents of the comparison group was 1.65 (SD=0.72), which was significantly higher than that of the parents of the patients (t=5.48, df=22, p=0.0001). Ten percent of the comparison subjects were left-handed, 1% showed mixed handedness, and 89% were right-handed.
Magnetic Resonance Imaging (MRI)
Subjects were scanned on a 1.5-T Signa scanner located at the National Institutes of Health Clinical Center, Bethesda, Md. A three-dimensional, spoiled-gradient, recalled echo in the steady state imaging sequence (TE=5 msec, TR=24 msec, flip angle=45°, acquisition matrix=192×256 pixels, number of excitations=1, field of view=24 cm) was used to obtain T1-weighted images with a slice thickness of 1.5 mm in the axial and sagittal planes and 2.0 mm in the coronal plane. To control for head position, external markers (vitamin E capsules) were used—one in the meatus of each ear and one taped to the left inferior orbital ridge—ensuring that all three were visible in the same axial reference plane. Rotation in the other plane was controlled by aligning the subject's nose to the 12:00 o'clock position. Foam padding was placed on the side of the head to minimize head movement.
Imaging data collected in Bethesda for the 24 patients and 95 comparison subjects were sent by File Transfer Protocol to the Imaging Processing Laboratory at the Mental Health Clinical Research Center at the University of Iowa. All postacquisition processing was done with the use of a locally developed family of software called BRAINS. Details of the image analysis have been published elsewhere (
27–
30). The MRI data were converted to a three-dimensional data set with the use of BRAINBLAST, a voxel-processing program that does surface and volume rendering. Next, the brains were resampled in 1-mm slices, and the resampled slices were then visualized in multiple planes simultaneously. This allowed for a thorough, millimeter-by-millimeter inspection of the entire brain in all three orientations (coronal, sagittal, and axial).
Rating of CSP
Brains were identified by a number only. Thus, the rater was blind to the diagnosis, sex, and age of the subjects. All brain images were visually inspected by one of us (P.N.), who is well versed in MRI of developmental brain anomalies. With the exception of our most recent study (
7), all previous studies assessing CSP have used a qualitative assessment of the anomaly (absent, small/questionable, moderate, large). In the present study we used a more quantitative approach, measuring the size of the cavum by its appearance in consecutive coronal 1-mm slices: a rating of 1 represents a cavum seen in only one coronal slice, a rating of 2 represents a cavum seen in two consecutive coronal slices, and so on. Since the images are 1 mm without gaps, the rating is a reflection of the actual anterior-to-posterior length of the cavum, although partial voluming renders this an approximation only. For example, a CSP with a rating of 5 would be approximately 5 mm long.
Reliability for rating the CSP was established in our previous project with 100 subjects (
7). Agreement between the two raters was high at 79%, and for ratings about which the two raters disagreed, the difference was never more than 1 point.
In both the literature review and the results from our recent study on the frequency of CSP, it is clear that a small CSP is common in a large proportion of healthy subjects (
7,
31–
33) and is therefore considered to be a “normal variant.” It is not known exactly how large the CSP must be in order to reflect pathology, although it is clear that an overly conservative estimate may erroneously include a CSP that is in the upper range of the normal variant. The best estimate of the size of a normal-variant cavum is approximately 1–4 mm (
13,
34–
37). To allow some room for partial voluming and to eliminate “borderline-large” CSP, we defined enlargement of the CSP as length greater than 6 mm. All subjects were then placed in one of two groups: those with CSP enlargement (ratings greater than 6) and those without (ratings of 0–6).
Statistical comparisons between the two groups were done with chi-square analysis unless a cell had fewer than five subjects, in which case Fisher's exact test was used.
RESULTS
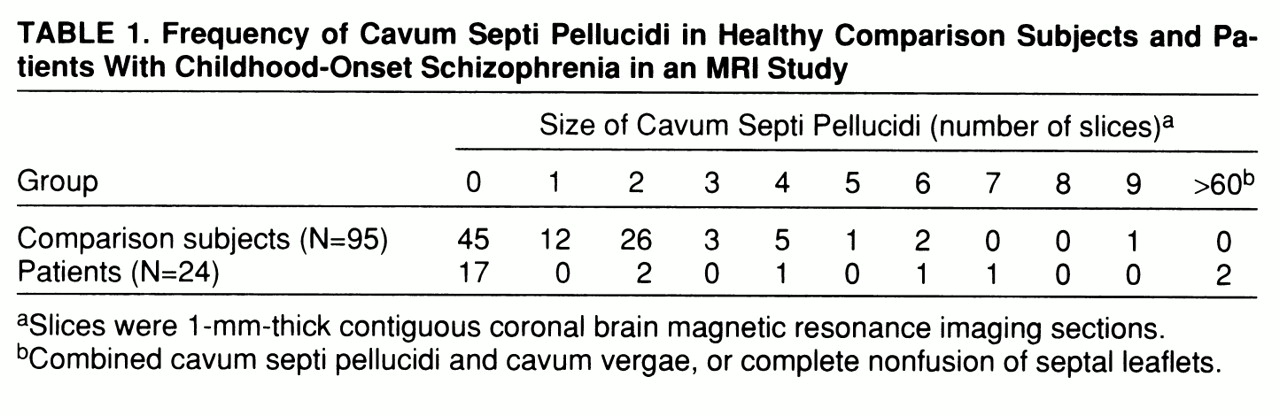
Table 1 shows the frequency of CSP in both groups. As has been documented by many studies as well as our own, a small CSP (fewer than six slices or 6 mm) was common in both the healthy comparison subjects and the patients. However, an enlarged CSP (more than six slices) occurred in only one (1.1%) of the 95 healthy subjects, an 11-year-old girl. In contrast, three (12.5%) of the 24 patients were found to have an enlarged CSP (p<0.03, Fisher's exact test).
Although not arbitrary, the definition of an enlarged CSP may not be exact. Two comparison subjects and one patient had a CSP 6 mm in size, which could be construed as borderline large. Therefore, we reran the analysis with the definition of an enlarged CSP as equal to or greater than six slices, thus including these borderline cases. The result was still significant, with three of the 95 comparison subjects having an enlarged CSP and four of the 24 patients having an enlarged CSP (p=0.03, Fisher's exact test).
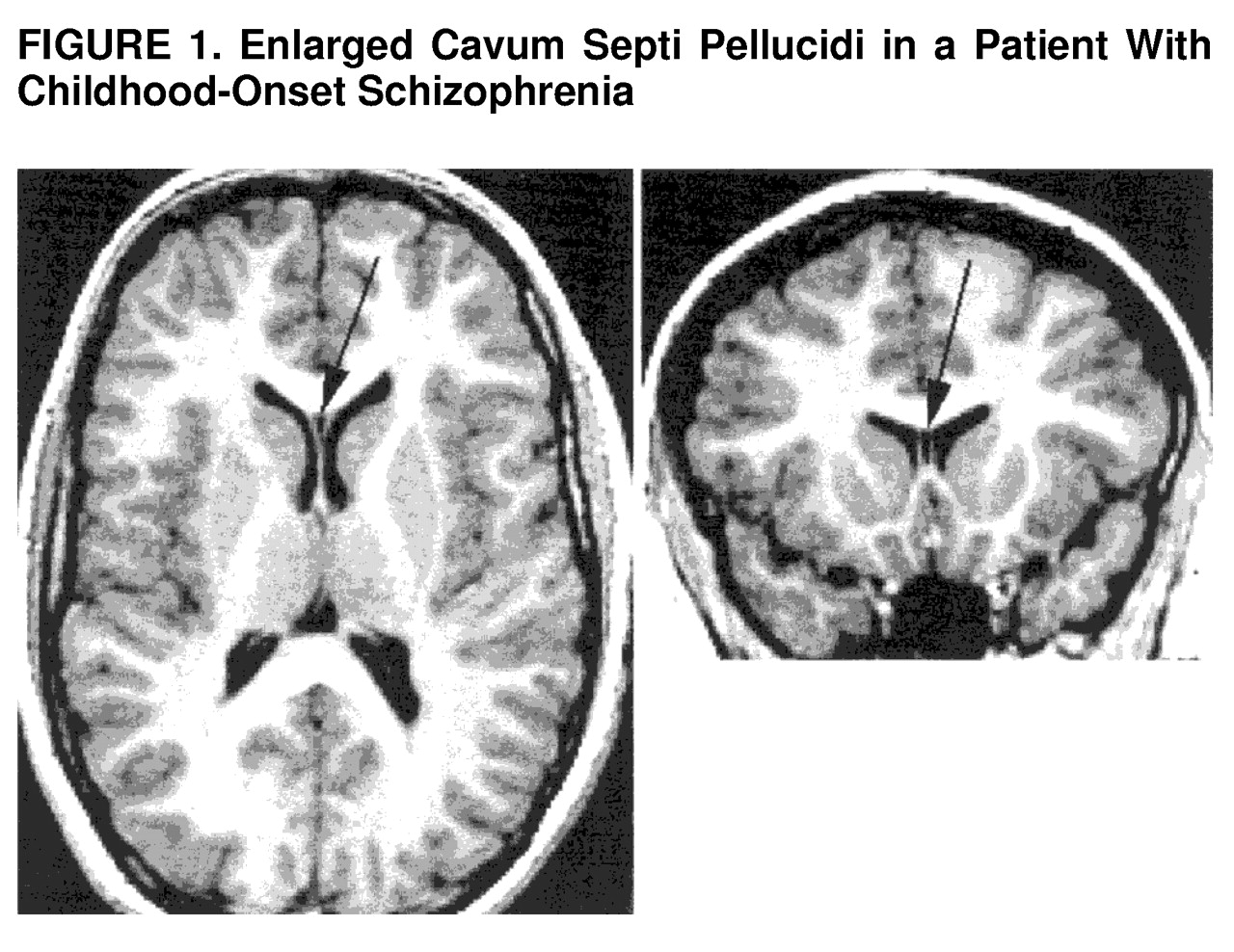
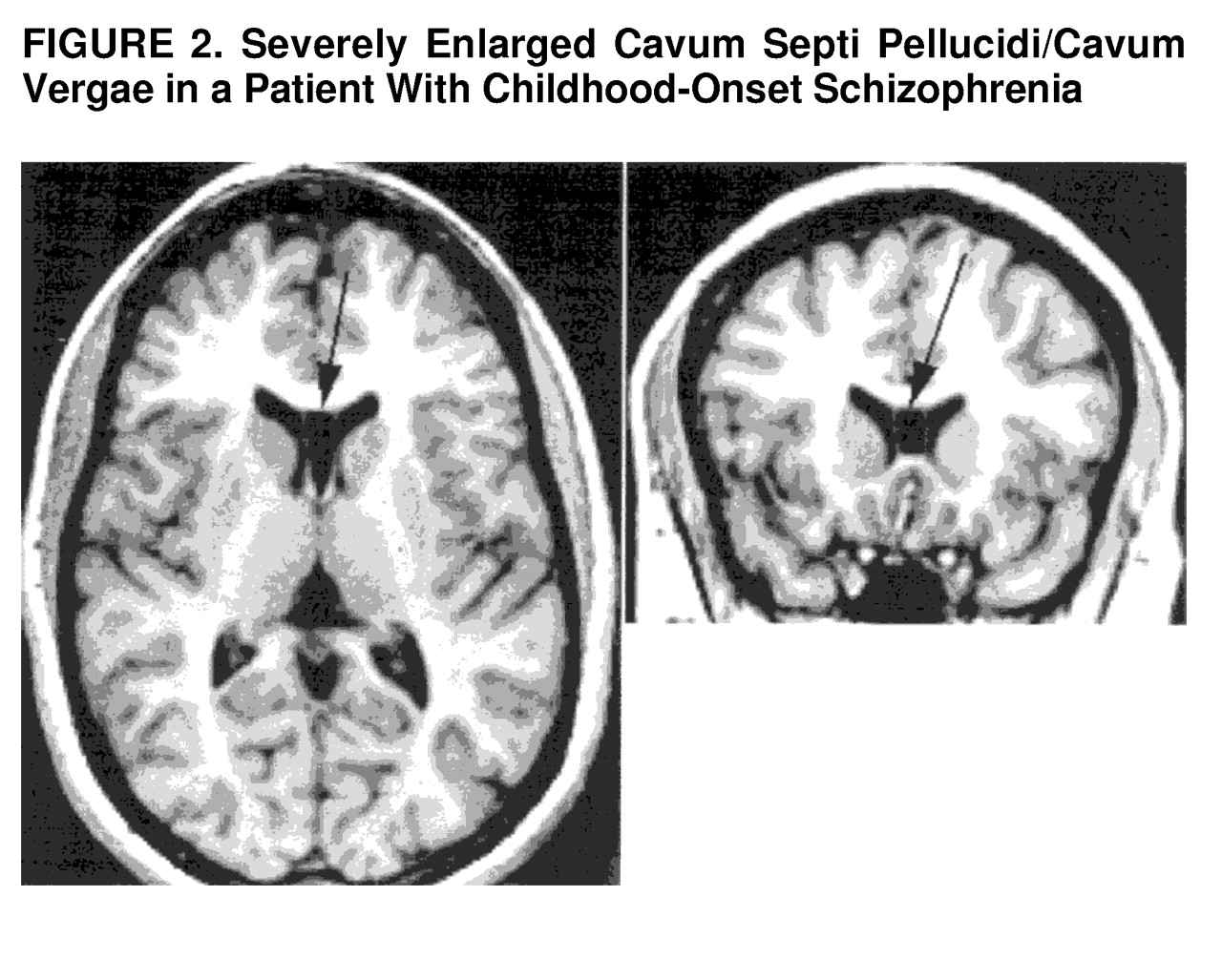
With regard to severity of the anomaly, of the three subjects with an abnormally large CSP, one (a 14-year-old boy) had a rating of 7 (
figure 1), but the remaining two (a 16-year-old girl and a 16-year-old boy) had complete nonfusion of the entire length of the septum pellucidum—an abnormality referred to as combined CSP and cavum vergae (CV) (
figure 2). These were reflected in ratings of more than 60 slices (the entire length of the septum pellucidum). Whereas CSP enlargement reflects an arrest of the normal fusion process of the leaflets of the septum pellucidum, a combined CSP and CV (CSP/CV) reflects not an arrest but total lack of any fusion process, a more severe anomaly.
DISCUSSION
In sum, the group of patients with childhood-onset schizophrenia was found to have an increased rate of a developmental brain anomaly—enlarged CSP—in comparison with the group of healthy subjects: 12.5% of 24 patients had the anomaly versus 1.1% of 95 healthy subjects. The rate of CSP enlargement in the healthy comparison subjects is similar to the rate we reported earlier in our adult subjects (1.3%) (
7). In addition, the 12.5% frequency of CSP enlargement in the current patient group is very similar to the rate we reported in our adult-onset group (10.9%, N=6 of 55) (
7). It is, however, lower than rates reported by DeLisi et al. (44.8%) (
10), Degreef et al. (21%) (
8), and Jurjus et al. (25%) (
38) in three MRI studies with adult-onset schizophrenic subjects. This is most likely due to a difference in the definition of CSP. The DeLisi, Degreef, and Jurjus studies did not exclude small CSP, thus including in their analysis any CSP in the range of 1–6 mm in length, which is common in both patient and comparison groups and is considered normal anatomic variation. In our study group, the inclusion of all sizes of CSP raises the number found in patients to seven of 24, or 29.2%, a proportion much more in line with the findings of the other MRI studies. However, to define a pathological CSP or an abnormally enlarged CSP, small or normal-variant CSP must be excluded. Shioiri et al. (
11) found the exact frequency of an enlarged CSP in their study that we found in the current study (12.5%) when they excluded subjects with a small CSP.
Possibly more important than the frequency of the anomaly is the severity of the anomaly. The size of the CSP in the one healthy comparison subject with the anomaly was nine slices or 9 mm, whereas two of the three patients with the anomaly had a severe enlargement (more than 60 slices or 60 mm), representing total lack of fusion of the septal leaflets at any time—a combined CSP/CV. The rate of CSP/CV in the current study was 8.3% (N=2 of 24). Comparison of this rate with the rate reported in the literature on adult onset is difficult, since the reporting of CSP/CV is inconsistent. However, the frequency in our childhood-onset group appears somewhat higher: Degreef et al. (
8) reported 3.2% (N=2) of 62 adult patients with combined CSP/CV; our previous study (
7) found no CSP/CV in 55 adults. Jurjus et al. (
38), Shioiri et al. (
11), and DeLisi et al. (
10) did not report CSP/CV in any of their subjects. Since CV almost invariably occurs in association with a severely enlarged CSP, we used the frequency of severe enlargement in these three studies for comparison instead: in the study by Jurjus et al. (ratings of moderate and severe CSP combined), 6.6%; in the study by Shioiri et al., 5.0% (N=2 of 40); in the study by DeLisi et al., none of 85. A study by Scott et al. (
4) reported that five (9.6%) of 52 patients with schizophrenia had CSP/CV. With the exception of that study, the rate of either severe CSP or CSP/CV in the adult-onset groups appears to be lower than the rate of this anomaly in our childhood-onset group.
One important limitation to the interpretation of these results is that the childhood-onset group was selected for lack of response to treatment and may not be representative of the total population with childhood schizophrenia. Investigators have speculated that patients who are not responsive to medication may have more brain abnormalities (
39). However, other childhood-onset study groups have indeed been described as severely ill (
17,
40–
43). The phenomenologic characteristics and premorbid histories of the current group do not differ much from the “typical” childhood-onset groups previously described. The current study group was quite ill, yet this level of severity may in fact be representative of the childhood-onset form of schizophrenia.
Another limitation is that the social class of the parents of the comparison subjects was significantly higher than the social class of the parents of the patients. Although social class may affect brain morphology through developmental mechanisms (e.g., nutrition, illnesses, and education), it is not known whether specific anomalies such as CSP can be attributable to factors such as social class. However, both in the current study's patient and comparison groups and in the adult patient and comparison groups previously studied by our laboratory (
7), there appeared to be no relation between parental socioeconomic status and CSP size: the Spearman correlation was 0.03 (N=23, p=0.86) in the childhood-onset patient group and 0.11 (N=72, p=0.35) in the healthy childhood comparison group; the Spearman correlation was 0.18 (N=55, p=0.18) in the adult patient group and 0.003 (N=74, p=0.97) in the healthy adult comparison group. Therefore, it seems unlikely that the differences in parental socioeconomic status between the patient and comparison groups could account for the findings of increased CSP in the patients.
These findings lend further support to the theory that the childhood-onset form of schizophrenia is indeed along the same continuum and has the same pathophysiology as the adult-onset form. As would be predicted by this theory, the same type of developmental anomaly seen in the adult-onset form, enlargement of the CSP, is also found in the childhood-onset form. In addition, there is a striking similarity in the rates of CSP enlargement in the present childhood-onset group and the adult-onset study groups, a finding that would be quite unexpected if the two forms of the illness had separate and distinct etiologies.
Although the type of anomaly and the frequency of the anomaly are similar in the childhood-onset and adult-onset forms of schizophrenia, the childhood-onset form may manifest a more severe CSP enlargement, as was suggested in this study. In addition, in the previous study of brain morphology in childhood-onset schizophrenia (of which the current study group is a subset), comparison of effect sizes with those in adult studies indicated a greater relative reduction in total cerebral volume and in midsagittal thalamic area for the childhood-onset patients (
44). This finding supports the neurodevelopmental models of schizophrenia, which suggest that the more severe the brain dysgenesis, the earlier the onset of psychotic symptoms.
What are the etiologic implications of enlargement of the CSP? There is now a considerable amount of evidence to support the notion that brain dysgenesis may play an important role in the pathogenesis of schizophrenia (
45–
47). CSP can be considered a midline neurodevelopmental anomaly involving a limbic system structure. The normal brain maturation process by which the two leaflets of the septum pellucidum are fused is directly related to the rapid growth of the hippocampal alvei (the thin layer of white matter on the ventricular surface, which consists of fibers that enter and leave the hippocampal formation) and the corpus callosum (
13). This suggests that dysgenesis of either the hippocampus or the corpus callosum could possibly lead to an arrest of the fusion process, causing a larger-than-normal CSP to persist. In the literature on adult patients, developmental abnormalities of both the hippocampus (
48–
50) and the corpus callosum (
4,
5,
51–
53) have been reported. Quantitative assessment of brain morphology of a subset of the current study group failed to show any significant decrement in the volume of temporal lobe structures (
54). However, the investigators did find that the patients lacked the normal hippocampal asymmetry (right greater than left). This finding is similar to other reports in the literature on adult patients that suggest a relationship between asymmetry of temporal lobe structures and CSP. DeLisi et al. (
10) reported a trend association between the severity of CSP and reduction in asymmetry (right greater than left) in the temporal cortex. A report by our group (
55) compared patients with CSP to patients without CSP and found that those with a large CSP had more pronounced temporal lobe volume asymmetry (right greater than left). Although the direction was reversed in that study (more pronounced right-greater-than-left asymmetry rather than a reduction of right-greater-than-left asymmetry), these findings suggest that it may not be temporal lobe dysgenesis that is associated with CSP, but
lateralized temporal lobe dysgenesis that contributes to disruption of fusion of septum pellucidum leaflets.
The presence of CSP is most likely indirectly related to the underlying pathology of schizophrenia. It can be considered a marker of limbic system dysgenesis, a forme fruste of midline abnormalities, or both. It is yet another clue that leads us to further investigation of early brain development and of how aberrations in normal brain genesis are manifested in neuropsychiatric illnesses. More important, additional study of developmental anomalies in the childhood-onset population may further our understanding of the role of these anomalies in the pathology of schizophrenia.