In Greek mythology, Atropos was responsible for severing the thread of life. Indeed, in older patients, the atropinic effects of medications may be life threatening and compromise quality of life (
1). The total anticholinergic burden of medication has been quantified by measuring serum anticholinergicity. Serum anticholinergicity has been associated with delirium (
2) as well as more subtle impairments in memory (
3), attention, and self-care capacity (
4). The selective serotonin reuptake inhibitors have greatly advanced the pharmacotherapy of late-life depression because of their apparent absence of antimuscarinic effects. Nonetheless, concern exists regarding paroxetine. For example, Richelson (
5), using in vitro radioreceptor analyses, reported that paroxetine has approximately one and a half times the affinity that nortriptyline has for muscarinic receptors and is comparable to imipramine in its affinity for these receptors. This has not been consistent with our clinical experience or with other published in vitro data (
6). Consequently, we were interested in comparing anticholinergicity in sera obtained from older patients treated with these two medications.
METHOD
The subjects for this study were 61 depressed patients whose mean age was 73.2 years (SD=7.5, range=60.4–95.1). Written informed consent was obtained from each subject after the procedures had been fully explained. Patients were then randomly assigned to double-blind treatment with either nortriptyline (target plasma level=100 ng/ml) (N=30) or paroxetine (20 mg or 30 mg if the response was minimal after 2–4 weeks) (N=31). Plasma was obtained weekly for measurement of the concentrations of paroxetine and for nortriptyline and its E-10 and Z-10 hydroxy metabolites. Serum was sampled for serum anticholinergicity at baseline and after 1, 4, and 6 weeks. All blood samples were acquired 12–15 hours after the evening dose of medication.
We used the well-established radioreceptor binding assay of Tune and Coyle (
7) to analyze serum anticholinergicity; results are expressed in picomoles of atropine equivalents. This method quantifies the competitive displacement of tritiated 3-quinuclidinyl benzilate from rat striatal and forebrain receptors. Atropine at various concentrations was used to calibrate the assay. The standard curve is linear (r=0.99) from 0.10 to 5.0 pmol, and the interassay coefficient of variation over these concentrations ranged from 4.3% to 11.5% (based on six assays). Antidepressant drug and metabolite concentrations were measured by high performance liquid chromatography with ultraviolet detection (
8,
9). Side effects were assessed each week by using the UKU Side Effects Rating Scale (
10).
The absolute change from baseline in serum anticholinergicity was compared between the drug groups first by a two-group, three-time-point, repeated measures analysis of variance (ANOVA). Post hoc pairwise comparisons following the ANOVA were then done with Wilcoxon rank sum tests at each time point. Spearman correlations were calculated to examine the relation of drug plasma levels to serum anticholinergicity. The association of drug assignment with UKU-assessed side effects was assessed with Fisher exact probability because UKU ratings are categorical. Changes in heart rate were tested with the Wilcoxon signed ranks test. SAS software (
11) was used to perform all analyses.
RESULTS
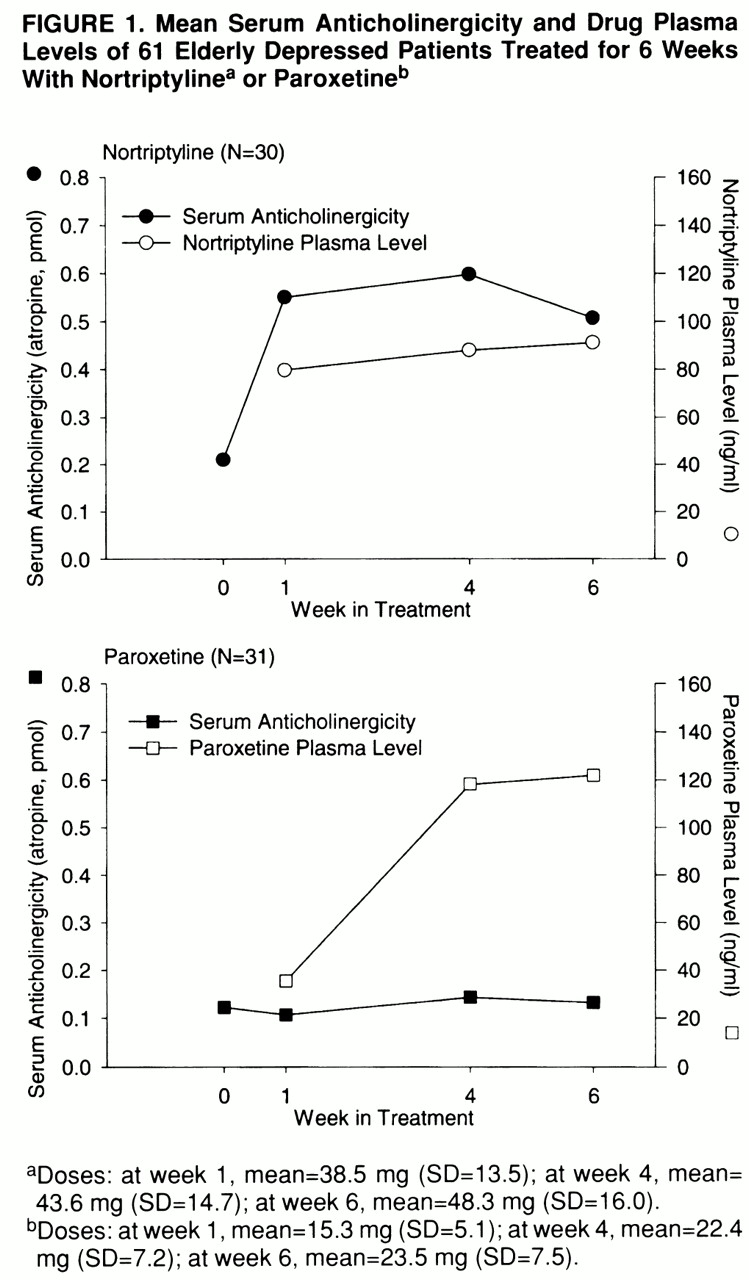
Before treatment, there were no significant differences between drug treatment groups in demographic or clinical characteristics or in medical burden. Examining the changes in serum anticholinergicity with treatment, we found a significant difference between the drug groups (F=14.25, df=1,59, p=0.004) but no significant group-by-week interaction. After 1 week of treatment, the median change in serum anticholinergicity for the nortriptyline group was 0.28 pmol atropine equivalents (range=–0.2 to 2.23 pmol), compared with a median change of 0 pmol atropine equivalents (range=–0.56 to 0.18 pmol) for paroxetine-treated patients (z=4.2, p=0.0001). The changes from baseline in serum anticholinergicity continued to be significantly greater for the nortriptyline-treated patients than for the paroxetine group: at week 4 the median for the former was 0.31 pmol (range=–0.32 to 1.32), compared with 0 pmol (range=–0.65 to 1.09) for the latter (z=3.6, p=0.0004), and at week 6 the median for the nortriptyline group was 0.18 pmol (range=–0.18 to 1.23), compared with 0 pmol (range=–0.59 to 0.77) for the paroxetine group (z=2.6, p=0.01).
Mean weekly drug doses, drug plasma levels, and serum anticholinergicity for the patients treated with nortriptyline and paroxetine are shown in
figure 1. Plasma concentrations of nortriptyline at 6 weeks (mean=91 ng/ml, SD=41) were significantly correlated with the changes in serum anticholinergicity from baseline to week 6 (r=0.54, df=20, p=0.01). Plasma concentrations of nortriptyline's hydroxy metabolites (mean=222 ng/ml, SD=74) and plasma concentrations of paroxetine (mean=122 ng/ml, SD=117) after 6 weeks of medication were not correlated with changes in anticholinergicity.
Nortriptyline-treated patients had significantly higher UKU scores for dry mouth (p=0.004, Fisher's exact test) and tachycardia or palpitations (p=0.01, Fisher's exact test) but not for complaints of constipation or urinary retention. Heart rates, as determined by ECGs at baseline and 6 weeks later, increased significantly by an average of 5 bpm in the nortriptyline-treated patients (p=0.03, Wilcoxon signed ranks test). In the paroxetine group, heart rate decreased significantly by 6.5 bpm (p=0.01, Wilcoxon signed ranks test).
DISCUSSION
In contrast to the in vitro results of Richelson (
5), but in agreement with the findings of Cusak et al. (
6), our findings in older patients suggest that, at therapeutic doses, paroxetine has approximately one-fifth the anticholinergic potential of nortriptyline. The significant correlation of plasma nortriptyline concentrations with serum anticholinergicity suggests a dose-response relationship that was not apparent for paroxetine. If paroxetine had significant anticholinergicity, one would also expect anticholinergic side effects in paroxetine-treated patients. This was not found in our study, where side effect scores for dry mouth and tachycardia or palpitations were significantly increased only for the patients given nortriptyline. The noradrenergic actions of nortriptyline may have also exacerbated both of these side effects.
Discrepancies between in vitro and ex vivo anticholinergic activities may arise because of differential effects of protein binding or the generation of active metabolites. Although both paroxetine and nortriptyline are highly protein bound (>95%), a slight difference of only a few percent could substantially alter the relative proportions of available free drug. Moreover, the contributions of nortriptyline's hydroxy metabolites need to be considered. In our study, these metabolites were present in greater concentrations than nortriptyline. The hydroxylated metabolites of nortriptyline are known to be substantially less protein bound than their parent and to possess anticholinergic activity (
8). In contrast, paroxetine does not appear to have active metabolites with anticholinergic potential.