Every known antipsychotic blocks dopamine D
2 receptors. While this does not prove a causal relationship between D
2 blockade and antipsychotic response, the association between the two is undeniable. Thus, it is of obvious interest to compare the relative D
2 receptor occupancies of the available antipsychotics. Farde and colleagues (
1) proposed that one needs a “threshold” of D
2 occupancy to induce antipsychotic response. Prospective studies have largely confirmed this notion, although it is unclear whether this threshold is 60% or 70% and whether the threshold for inducing response is the same as the one for maintaining it (
2,
3). Nonetheless, there is a mechanism of antipsychotic response that relies on D
2 occupancy alone (
4). Furthermore, it has been noted that as D
2 occupancy increases, especially as it rises above 80%, the incidence of extrapyramidal side effects increases (
1). Elevation of prolactin levels may also show a threshold relationship with respect to D
2 occupancy (
5,
6, and unpublished data from our laboratory). While D
2 occupancy may be necessary for response, it is not always sufficient, as there are patients who do not respond despite adequate D
2 occupancy (
7). However, it would be fair to claim that D
2 occupancy provides a reliable (and perhaps the best) pharmacological predictor of response to antipsychotic medication, extrapyramidal side effects, and elevation of prolactin levels.
It has been suggested that drugs may demonstrate atypical clinical features (i.e., low extrapyramidal side effects and prolactin elevation) if they have at least a 10 times higher affinity for serotonin 5-HT
2 receptors than D
2 receptors in vitro (
13,
14). In patients this translates into a preferential occupancy of 5-HT
2 receptors as opposed to D
2 receptors (
15). Clozapine, risperidone, and olanzapine all show a higher affinity for 5-HT
2 receptors than D
2 receptors in vitro, but the amount by which 5-HT
2 exceeds D
2 is unclear. In animal tissue or cloned human receptors, clozapine shows a much higher 5-HT
2/D
2 ratio (20 times higher affinity for 5-HT
2 than for D
2) than either risperidone (11 times) or olanzapine (12 times) (
16). On the other hand, occupancy measurements in rats following subcutaneous injection showed that risperidone had the highest 5-HT
2/D
2 affinity ratio (19 times) as compared with clozapine (5.1 times) and olanzapine (7.5 times) (
16). Therefore, a second purpose of this study was to systematically compare the 5-HT
2 occupancies and the 5-HT
2/D
2 occupancy ratios in patients.
Several previous reports have presented data on one or two of these antipsychotics separately. However, these studies used different imaging modalities (positron emission tomography [PET;
17–
21] or single photon emission computed tomography [SPECT;
22,
23]); different radioligands ([
11C]raclopride [
17–
20], [
11C]- NMSP [
21], or [
123I]IBZM [
22,
23]); and different image analysis models (cerebellum as reference [
17–
21] or frontal cortex as reference [
22,
23]). As a result, it is not valid to compare the occupancy of, say, risperidone obtained with [
123I]IBZM SPECT to that of clozapine derived by using [
11C]NMSP PET. It is essential, when comparing drugs, that the data be obtained on all of the drugs in the same fashion. Furthermore, dose of a drug is a central determinant of its receptor occupancy, and some previous studies have compared occupancies without adequately controlling for dose (
23). Any systematic comparison across drugs must compare not just the occupancy but also the dose-occupancy relationships over the clinically relevant dose range. To provide the first such comparison, we present data on the relation between dose, plasma level, and 5-HT
2 and D
2 occupancies in 44 patients with schizophrenia who were receiving a wide range of steady-state doses of clozapine, risperidone, and olanzapine.
METHOD
The procedures for this study were approved by the Human Subjects Review Committee of the University of Toronto. Patients participated after receiving detailed written information about the study and signing a consent document. Patients receiving ongoing treatment in our inpatient and outpatient clinic were recruited over a period of 4 years. We studied 44 patients (36 male and eight female) aged 19–54 years; 43 had a DSM-IV diagnosis of schizophrenia, and one had a diagnosis of atypical psychosis. Sixteen patients were receiving risperidone (2–12 mg/day), 17 were receiving olanzapine (5–60 mg/day), and 11 were receiving clozapine (75–900 mg/day). The patients were not randomly assigned to the three drugs, and the limitations of this are discussed later. Most of the patients were chosen from a clinic population so as to provide a representative range of doses for each drug, which is a sufficient study design for obtaining the in vivo 5-HT2/D2 profile of the three drugs in patients. However, because of inadequate prospective control over drug and dose assignment and the fact that patients were in various degrees of remission at the time of scanning, the design was limited in its ability to obtain reliable associations between receptor occupancy and clinical outcome. Nonetheless, to ensure a valid comparison of the action of the three drugs on the receptors, the patients in this report conformed to the following criteria.
1. All patients were on multiple-dose, steady-state levels (at least 5 days on the particular dose) of the medication for which they were assessed.
2. All patients were free of any confounding major medical or neurological illness. None of the patients had received any depot antipsychotics in the year before this PET examination, and none had received any other antipsychotics for a minimum of 14 days before the PET examination. None of the patients had concurrent substance abuse or substance dependence (nicotine excepted).
3. Patients were not receiving any other psychotropic medication that might confound the findings for D
2 occupancy, with the exception of benzodiazepines and/or an antiparkinsonian agent, as indicated in
table 1. It has been shown that the addition of lorazepam does not alter the measurement of D
2 occupancy with [
11C]raclopride (
24).
4. All patients received a scan for dopamine D2 receptors, and most received a scan for serotonin 5-HT2 receptors. All patients had their [11C]raclopride PET examinations done 12–13 hours after their last nightly drug dose (the usual scan start was at 9:00 a.m.). The [18F]setoperone PET examination for 5-HT2 receptors, if done, followed the [11C]raclopride examination on the same day and started 14–15 hours after the last nightly drug dose.
PET data on some of the patients have appeared in previous reports (
19,
20); these patients are identified in
table 1. To carry out a systematic comparison, data on all of the subjects in the study were analyzed (those reported before were reanalyzed) with the methods described below, and all patient data were compared with the same baseline data to eliminate any across-drug bias. As a result, there may be a slight variation in the occupancy reported here and that reported before.
The PET scans to estimate D
2 occupancy were obtained after the injection of 10 mCi of high specific activity [
11C]raclopride (300–1600 Ci/mmol) according to a bolus-plus-infusion protocol. The scanning methods used have been described in a previous report in this journal (
2). The pertinent aspects are the following. Striatal and cerebellar regions of interest were drawn on two contiguous PET slices on a composite PET image with reference to a coregistered magnetic resonance imaging (MRI) scan (GE Signa 1.5-T scanner; T
2-weighted spin-echo sequence coregistered to the PET scan with a surface-matching algorithm). An estimate of the D
2 binding potential (D
2 BP) of raclopride (which represents the B
max/K
d of [
11C]- raclopride for D
2 receptors, where B
max is the total number of receptors available to a ligand, and K
d is the affinity of the ligand for the receptors) was obtained from the ratio of the striatum to the cerebellum during the 30- to 75- minute interval, a period of prolonged pseudo equilibrium afforded by the bolus-plus-infusion protocol. As used in our laboratory, this method yields a test-retest standard deviation of 6% and has been standardized to have an interrater and intrarater reliability, as measured by the intraclass correlation coefficient (ICC-III), of >0.95.
To estimate receptor occupancy, we used an age-corrected baseline value derived from a pool of 16 normal subjects and 12 antipsychotic-naive patients with schizophrenia. Since the illness had no statistically discernible effect on D
2 receptors as measured with [
11C]raclopride in this sample (F=0.66, df=1, 25, p=0.42) and in a previous report by others (
25), data from the antipsychotic-naive patients and normal subjects were pooled to provide a more robust effect of age on D
2 binding potential. Drug-induced D
2 receptor occupancy was determined as (D
2 BP
Base–D
2 BP
Drug)/(D
2 BP
Base), where D
2 BP
Base is the age-corrected D
2 baseline binding potential, and D
2 BP
Drug is the D
2 binding potential for patients taking the antipsychotic drug. The absence of the patient’s own baseline values introduces a potential error; the error as calculated on the basis of variance in the data from antipsychotic-naive persons is expected to vary from 0% to 9% for patients with 50% occupancy and from 0% to 4% for patients with 80% occupancy (
26).
The 5-HT
2 scans were obtained with the use of a bolus injection of 5 mCi of high specific activity [
18F]setoperone (360–6210 Ci/mmol), according to the method developed and standardized by Blin et al. (
27,
28). The 5-HT
2 occupancy was determined in the prefrontal cortex regions of interest drawn on the [
18F]setoperone scan with reference to a coregistered MRI (as described above). An index of the 5-HT
2 receptors was obtained from the prefrontal cortex/cerebellar ratio over the 65- to 90-minute time period. The cerebellum is practically devoid of 5-HT
2 receptors (
29), and studies in baboons as well as humans report no displaceable [
18F]setoperone binding in this region (
27,
28,
30). It can be shown that at a time when the binding of the radioligand is at pseudo equilibrium, the prefrontal/cerebellum ratio represents 5-HT
2 BP + 1 (
31). The details of this method as applied here have been described elsewhere (
32). We have shown that this method yields an average test-retest deviation of 6%–7% and an acceptably high interrater reliability (ICC-III >0.95) (
32).
Since these patients were already receiving treatment, it was not possible to measure their baseline 5-HT
2 binding potential. In the absence of this baseline, we used the age-corrected 5-HT
2 binding potential obtained from 11 antipsychotic-free patients with schizophrenia and 26 age-matched normal subjects. The pooling of normal subjects and patients results in a more robust age-corrected regression and is justified because there was neither a main effect of diagnosis (F=1.20, df=1, 33, p=0.28) nor a significant effect of diagnosis on the age regression (F=0.59, df=1, 33, p=0.45) (
33). Occupancy was calculated in the same way as for dopamine D
2 occupancy.
The patients had blood drawn at the time of the D
2 PET scan; the samples were analyzed in batches by drug. Clozapine plasma levels were measured by means of high-performance liquid chromatography with electrochemical detection by a method based on previously published techniques (
34). Risperidone plasma levels were measured by means of a radioimmunoassay for the active moiety, which reflects both risperidone and 9-OH risperidone, by the Janssen Research Foundation, Beerse, Belgium (
35). Olanzapine plasma levels were measured by means of high-performance liquid chromatography with electrochemical detection by BAS Analytics (West Lafayette, Ind.).
Since the patients were, by design, taking the drugs at different dose and plasma levels, a direct comparison of the occupancies without reference to dose is not very informative. To provide a valid comparison, we characterized the dose-occupancy relationship for each drug and compared these profiles across the three drugs. The expected relationship between a drug level and occupancy of the receptors should conform to a rectangular hyperbola, assuming bimolecular (drug-receptor) interactions and negligible occupancy by the endogenous ligand. A rectangular hyperbola, in this context, is defined by
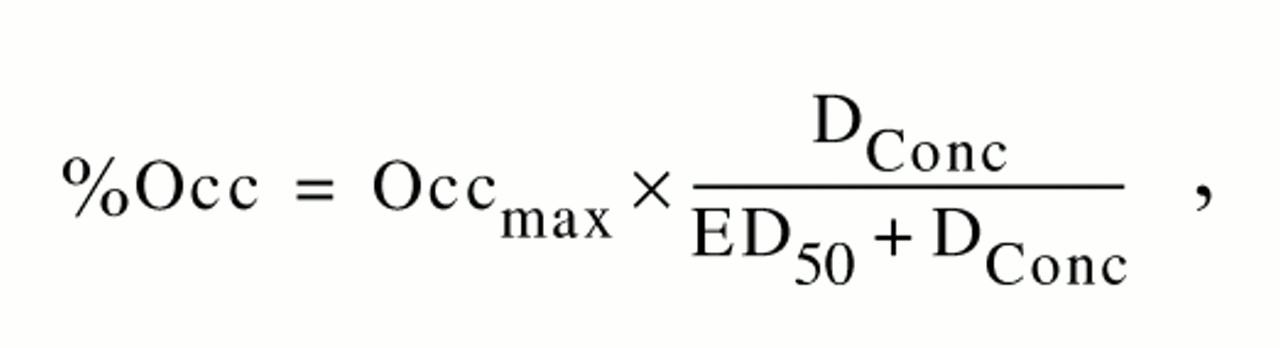
, where %Occ is the observed occupancy, Occ
max is the theoretically maximum occupancy for that particular drug, D
Conc is the amount of the drug (expressed either as dose or plasma level), and ED
50 is a constant equivalent to the amount of the drug required to occupy 50% of Occ
max. If there is reason to believe that all of the [
11C]raclopride can be displaced by the drug in question, Occ
max can be replaced by 100 in equation 1. In its truest form, D
Conc should represent the concentration of the drug in the synapse. Since there is no easy way to measure the synaptic concentration of the drug in patients, one can use dose and plasma level as functional surrogates. However, it should be kept in mind that dose and plasma level only indirectly reflect synaptic concentrations. Noncompliance with the medication regimen or changes in metabolism may lead to different plasma levels for a given dose, and there may be significant differences in the levels of the freely available drug in the synapse if there is a change in protein binding. The observed receptor occupancy was related to the administered dose and measured plasma level with the use of equation 1 implemented in SPSS for Windows (SPSS Inc., Chicago).
RESULTS
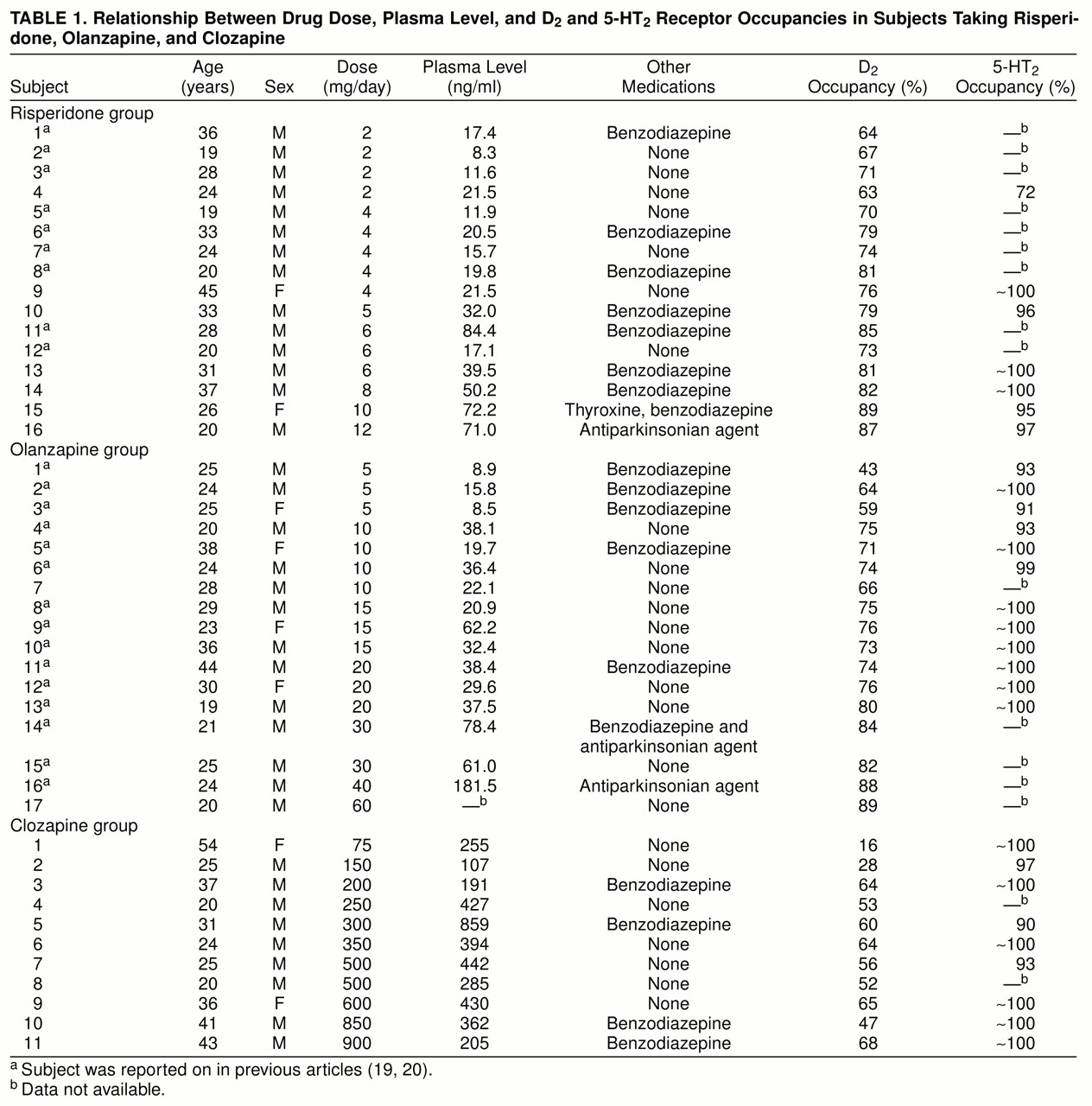
For all three drugs, increasing doses led to increasing plasma levels, consistent with expectations and affirming reasonable compliance (table 1). A simple comparison of the occupancies shows that clozapine had a significantly lower D2 occupancy than risperidone and olanzapine (main analysis of variance, F=13.05, df=2, 39, p<0.001; post hoc comparison with risperidone: p<0.001; with olanzapine: p<0.001), which were themselves indistinguishable (Tukey’s honestly significant difference post hoc test, risperidone versus olanzapine: p=0.80).
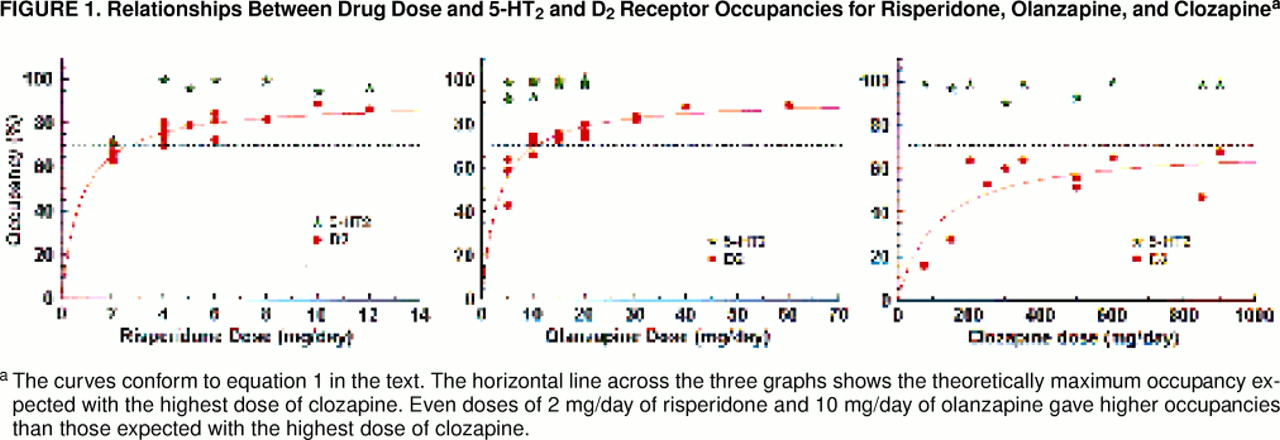
The D2 occupancies in response to increasing doses and plasma levels are presented in figure 1 and figure 2. In each case, a rectangular hyperbola provided a better fit (i.e., explained a greater degree of variance) than a simple linear fit. The parameters for the curves relating occupancy to dose were as follows: for risperidone, Occmax=91%, ED50=0.8 mg/day, with 77% variance explained; for olanzapine, Occmax=92%, ED50=3.2 mg/day, with 81% variance explained; for clozapine, Occmax=71%, ED50=112 mg/day, with 55% variance explained. The parameters for the curves relating occupancy to plasma level were as follows: for risperidone, Occmax=88%, ED50=3.2 ng/ml, with 59% variance explained; for olanzapine, Occmax=90%, ED50=6.4 ng/ml, with 84% variance explained; for clozapine, Occmax=68%, ED50=88 mg/day, with 18% variance explained. It is important to note that clozapine does not saturate the D2 receptors, and the theoretical maximum occupancy (Occmax) is 68%–71%. This is in contrast to risperidone and olanzapine, where we observed occupancies of up to 83% and where Occmax was 91% and 92%, respectively.
Curves relating dose and occupancy for risperidone and olanzapine had the same shape (a near-saturating rectangular hyperbola) and differed only in the dose axis. The amount of drug required to occupy 50% of the available D2 receptors is 3.2 mg/day for olanzapine and 0.8 mg/day for risperidone if the equation is fitted with two parameters (and 4.5 versus 1.2 mg/day if Occmax is assumed to be 100%). This suggests that D2-occupancy-equipotent doses of risperidone and olanzapine are in the ratio of 5:20 mg/day.
All three drugs showed a preferential occupancy of 5-HT
2 receptors versus D
2 receptors and the ability to completely occupy 5-HT
2 receptors, as measured by [
18F]setoperone (
figure 1 and
figure 2). The lowest doses tested—75 mg/day of clozapine, 2–4 mg/day of risperidone, and 5–10 mg/day of olanzapine—all led to more than 95% occupancy of frontal 5-HT
2 receptors in most patients. It should be noted that once occupancy goes beyond 90%, the signal available for analysis is less than 10%, and the precision of data beyond this point is limited. Since there were few patients who had midrange 5-HT
2 occupancies, it is not valid to try to fit the data to rectangular-hyperbola relationships. Nonetheless, clozapine possesses a greater degree of separation between its 5-HT
2 and D
2 occupancies than either risperidone or olanzapine (F=12.67, df=2, 25, p<0.0002; clozapine was different at p<0.05, according to Tukey’s post hoc unequal-N test, while risperidone and olanzapine were not different, p>0.10 ).
DISCUSSION
The data show that clozapine differs from risperidone and olanzapine in its D2 occupancy profile. Risperidone and olanzapine have similar profiles but differ in potency. All three of the antipsychotics are complete blockers of 5-HT2 receptors, although clozapine shows the greatest difference between its 5-HT2 and D2 occupancies. We discuss how these findings relate to previous published work, discuss the limitations of our results, and then present the implications of these findings for comparisons between antipsychotics.
Differences in ligand selection, imaging modality, and analysis procedures make comparisons of numbers obtained by different centers difficult. Therefore, our results are best compared with those reported by groups that have data, at least on one of the two receptors, on all three drugs. Pilowsky et al. (
23) compared the D
2 occupancies of clozapine, risperidone, and olanzapine using IBZM SPECT imaging. They concluded that the D
2 occupancy of olanzapine is statistically indistinguishable from that of clozapine and that the D
2 occupancy of risperidone is higher than that of both clozapine and olanzapine. Our data disagree with regard to olanzapine. The Pilowsky et al. study had several limitations. First, the spatial resolution, quantification, and signal/noise characteristics of IBZM SPECT imaging are inferior to those of [
11C]raclopride PET. Second, their study had a smaller total number of subjects (N=22, versus N=44 in this study), a restricted dose range (10–20 mg/day for olanzapine, while we report on 5–60 mg/day), and fewer subjects taking olanzapine (six, while we report on 17). Third, Pilowsky et al. compared the occupancies without reference to the dose of the drug—the average dose for olanzapine patients was 12.5 mg/day, while that for risperidone patients was 8.7 mg/day, a confounding factor that might explain their finding of a lower occupancy for olanzapine and a higher one for risperidone (see below). Finally, they found no relation between dose and occupancy, questioning the internal consistency of the data. The present study overcomes these limitations.
The Karolinska PET group has provided data on a series of patients taking 125–600 mg/day of clozapine (
18), four patients taking 6 mg/day of risperidone (
17), and three normal control subjects taking a single dose of 10 mg/day of olanzapine (
36). Our data on clozapine confirm theirs. We observed a maximal D
2 occupancy of 68%, and Nordstr�m et al. (
18) reported 67%; the theoretical maximum occupancy when plasma levels were related to D
2 occupancy was 68% in our study and 61% in theirs. Farde et al. (
17) reported that 6 mg/day of risperidone led to 75%–80% D
2 occupancy; our data show an average of 79% (range=73%–85%). Finally, on the basis of their study of single-dose olanzapine in normal volunteers, these investigators predicted that 10 mg/day of olanzapine should lead to 70% D
2 occupancy (
36), which is consistent with our observed average of 72% (range=66%–75%). Thus, our results are consistent with previous PET data and provide a more valid basis for comparison of the D
2 occupancies of the three drugs.
In addition, we made a comparison of the 5-HT
2 and D
2 occupancies of these drugs, which has never been reported before. It has been reported previously that low doses of clozapine lead to complete saturation of the 5-HT
2 receptors (
18). The new finding here is that risperidone and olanzapine also completely block 5-HT
2 receptors. The results refute the findings of the occupancy studies in rats done by Schotte et al. (
16) in which risperidone showed a greater separation of 5-HT
2 and D
2 occupancies. In our data, clozapine showed the greatest separation, with risperidone and olanzapine being similar.
Our study is not without its limitations. An important one is that the subjects were not randomly assigned to the drugs and to different doses of each drug. Since clozapine is used in patients who do not respond satisfactorily to other typical and atypical antipsychotics, there is a systematic selection bias here. While, clearly, a prospective randomized assignment to the three drugs would have been preferable, no study has yet been able to achieve this. Furthermore, while the clozapine patients do represent a more treatment-refractory group, there are no data to suggest that D
2 occupancy varies systematically as a function of lack of response; in fact, a previous study suggested that responders and nonresponders do not differ in D
2 occupancy (
7). [
11C]raclopride provides only striatal D
2 occupancy. The exact site of antipsychotic response is not known, but it is speculated that the mesolimbic D
2 receptors may be crucial. However, it is worth noting that a recent study comparing striatal to mesolimbic D
2 occupancy, using [
11C]raclopride and [
11C]FLB (
37), found no significant regional differences in D
2 occupancy between the striatum and the temporal cortex. It should be pointed out that preferential mesolimbic D
2 blockade is not a necessary precondition for mesolimbic selectivity. While there was no evidence for limbic selectivity of receptor blockade for any typical or atypical antipsychotics in ex vivo animal receptor occupancy studies (
16), there is clear evidence for preferential functional antagonism of the limbic as opposed to the striatal dopamine system with several of the atypical antipsychotics (
38).
Another limitation of our work, as of all the previous studies, is that the individual’s own pretreatment binding potential was not available; therefore, the estimate of occupancy carries an inherent error. However, this error is likely to be small and randomly distributed (
39). Furthermore, since the main focus of this study was the difference between the three drugs, all compared with the same baseline values, any such error is unlikely to account for the observed pattern of differences. Finally, it has been demonstrated in animals (
40 and suggested in humans (
5) that antipsychotics lead to an up-regulation of dopamine D
2 receptors. If so, a pretreatment baseline D
2 value may result in a lower estimate of D
2 occupancy. However, most animal data show that clozapine does not result in receptor up-regulation (
40,
41), while the situation with respect to olanzapine and risperidone is not clear. Even if up-regulation occurs, it is more likely with risperidone and olanzapine than with clozapine, and this would have decreased, rather than increased, our chances of finding the difference we report.
What do these results tell us about the pharmacological mechanisms of antipsychotic action? Farde and colleagues (
1) have suggested that one needs a threshold of D
2 receptor occupancy to obtain a satisfactory antipsychotic response. This threshold lies in the range of 65%–70% (
2,
4). It is interesting, then, that both risperidone and olanzapine become effective antipsychotics only at doses which cross these levels of D
2 occupancy. Doses of risperidone and olanzapine that do not reach these levels (i.e., <2 mg/day of risperidone and <10 mg/day of olanzapine) are not reliably antipsychotic in most clinical situations. Clozapine, on the other hand, is able to obtain an antipsychotic response with levels of D
2 occupancy (usually 30%–60%) that would be insufficient to cause antipsychotic response by themselves (
4). Thus, clozapine does not call on the typical D
2 occupancy mechanism for inducing antipsychotic response; risperidone and olanzapine do. This raises the possibility that despite some similarities in their receptor profile in vitro, the fundamental mechanism of response of clozapine may be different from that of olanzapine and risperidone in patients. While this question can ultimately be resolved only in a clinical arena in crossover studies, the data on D
2 occupancy provide a rationale for why patients who do not respond to risperidone/olanzapine may respond to clozapine and the other way around.
The 5-HT
2 receptor data show that all of these drugs are potent antagonists at that receptor. The fact that clozapine saturates 5-HT
2 receptors at such a low dose and that one continues to see an increasing response to clozapine in doses up to 350–400 mg/day (
42) makes it unlikely that 5-HT
2 is the primary source of the antipsychotic effect of clozapine. A similar conclusion was reached by Trichard et al. (
43) in their study of chlorpromazine and clozapine. It does not rule out the possibility that high levels of 5-HT
2 blockade may modulate or enhance the ability of D
2 blockade to induce response or to delay the onset of D
2-related side effects (
14,
15). However, any benefit provided by a mixed 5-HT
2/D
2 model must express itself in a narrow range. Patients taking risperidone and olanzapine who have high D
2 occupancies experience extrapyramidal side effects and prolactin elevation despite a concomitantly high 5-HT
2 occupancy (
19,
20).
These findings also raise interesting questions regarding the comparison of risperidone and olanzapine with each other and with typical antipsychotics. All of the currently published “pivotal” studies that have compared olanzapine and risperidone with the reference drug haloperidol have used 10–20 mg/day of haloperidol (
9,
10,
44). Thus, doses of olanzapine and risperidone that lead to 70%–80% D
2 occupancy have been compared with doses of haloperidol that result in more than 90% D
2 occupancy (
3,
19). It is increasingly being realized that the optimal dose of haloperidol for treatment of most patients may be less than 6 mg/day, while higher doses may result in greater extrapyramidal side effects without extra efficacy (
45). Since extrapyramidal side effects can also influence measurement of negative symptoms (
46), this raises an interesting question: would risperidone/olanzapine demonstrate superior benefits for extrapyramidal side effects, prolactin levels, and negative symptoms if they were compared with a typical antipsychotic that has the same D
2 occupancy? Such D
2-occupancy-matched studies will be very valuable for discerning the true components of atypicality.
These data are also of interest in interpreting the comparison between risperidone and olanzapine. In a recently published trial (
47), olanzapine, 17.2 mg/day, was compared with risperidone, 7.2 mg/day, and risperidone showed a higher propensity for extrapyramidal side effects and prolactin elevation. According to the equations derived from our data, the study compared an average of 77%–79% D
2 occupancy induced by olanzapine with an average of 82%–86% D
2 occupancy induced by risperidone. Because both extrapyramidal side effects and prolactin elevation are related to the amount of D
2 occupancy, and because as occupancy rises beyond 80%, extrapyramidal side effects become more prominent (
1), this raises the question of whether the higher incidence of extrapyramidal side effects/prolactin elevation with risperidone is a function of the choice of dose (and resulting D
2 occupancy) rather than a qualitative difference between the two drugs. On the other hand, since the design of the Tran et al. study (
47) permitted a clinical titration of the dose, it could also be true that olanzapine is able to muster a greater clinical response with a slightly lower level of D
2 occupancy (possibly because of the synergism of its multiple-receptor-blocking profile), and that if D
2-equipotent doses of risperidone and olanzapine were compared (e.g., 4–5 mg of risperidone versus 17.2 mg of olanzapine), risperidone might have lower extrapyramidal side effects/prolactin elevation, but olanzapine might then show superior efficacy.
In conclusion, this study provides the first systematic comparison of the D
2 and 5-HT
2 receptor occupancies of the three most commonly used atypical antipsychotics. Clozapine is able to induce an antipsychotic response with a D
2 occupancy lower than that of typical antipsychotics, and this may explain its freedom from extrapyramidal side effects and prolactin elevation. Risperidone and olanzapine become effective antipsychotics only when their D
2 occupancy is in the range obtained by low-dose typical antipsychotics (
2,
3). They occupy equal numbers of D
2 receptors when used in the dose ratio of 1:4 mg/day, respectively. All three drugs saturate the 5-HT
2 receptors at subtherapeutic doses, suggesting that 5-HT
2 occupancy alone is an unlikely explanation for their antipsychotic effects. The data also have implications for clinical comparison studies. Most studies comparing atypical antipsychotics with haloperidol have compared doses of risperidone/olanzapine that give 65%–80% D
2 occupancy with doses of haloperidol that give more than 90% D
2 occupancy. This may have biased these comparisons to produce higher extrapyramidal side effects and prolactin elevation with haloperidol. D
2-occupancy-matched trials of atypical antipsychotics versus haloperidol and atypical versus atypical antipsychotics would be more informative with regard to the true superiority of one drug over another and would also help in distinguishing the clinical role of receptors other than D
2.
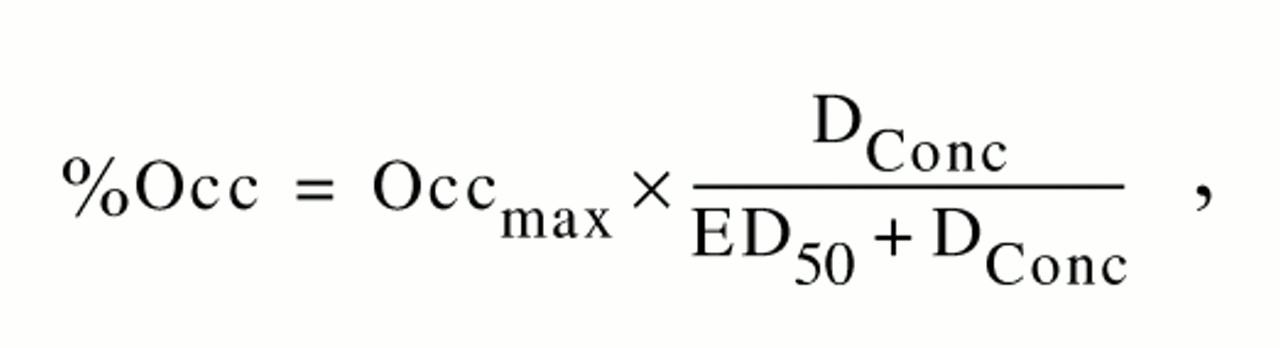 , where %Occ is the observed occupancy, Occmax is the theoretically maximum occupancy for that particular drug, DConc is the amount of the drug (expressed either as dose or plasma level), and ED50 is a constant equivalent to the amount of the drug required to occupy 50% of Occmax. If there is reason to believe that all of the [11C]raclopride can be displaced by the drug in question, Occmax can be replaced by 100 in equation 1. In its truest form, DConc should represent the concentration of the drug in the synapse. Since there is no easy way to measure the synaptic concentration of the drug in patients, one can use dose and plasma level as functional surrogates. However, it should be kept in mind that dose and plasma level only indirectly reflect synaptic concentrations. Noncompliance with the medication regimen or changes in metabolism may lead to different plasma levels for a given dose, and there may be significant differences in the levels of the freely available drug in the synapse if there is a change in protein binding. The observed receptor occupancy was related to the administered dose and measured plasma level with the use of equation 1 implemented in SPSS for Windows (SPSS Inc., Chicago).
, where %Occ is the observed occupancy, Occmax is the theoretically maximum occupancy for that particular drug, DConc is the amount of the drug (expressed either as dose or plasma level), and ED50 is a constant equivalent to the amount of the drug required to occupy 50% of Occmax. If there is reason to believe that all of the [11C]raclopride can be displaced by the drug in question, Occmax can be replaced by 100 in equation 1. In its truest form, DConc should represent the concentration of the drug in the synapse. Since there is no easy way to measure the synaptic concentration of the drug in patients, one can use dose and plasma level as functional surrogates. However, it should be kept in mind that dose and plasma level only indirectly reflect synaptic concentrations. Noncompliance with the medication regimen or changes in metabolism may lead to different plasma levels for a given dose, and there may be significant differences in the levels of the freely available drug in the synapse if there is a change in protein binding. The observed receptor occupancy was related to the administered dose and measured plasma level with the use of equation 1 implemented in SPSS for Windows (SPSS Inc., Chicago).