The development of functional connections between limbic structures and neocortex has long been considered critical to the evolution of complex emotional behaviors, although conclusive experimental evidence from studies of humans is relatively sparse
(1–
6). Clinical observation and personal experience nonetheless confirm the inseparable and often intrusive effects of transient changes in mood state on attention span, decisiveness, and other cognitive abilities, be it the fleeting sadness one feels on hearing a tragic news event or the more sustained feelings of melancholia and impaired concentration that accompany intense grief. The chronic and more insidious disruption of attentional processing observed in depressive illness
(7,
8)—an extreme instance of a sustained altered mood state—further suggests common neural pathways mediating both the normal and pathological interactions of these behaviors.
Comparative anatomical studies provide material support for these hypotheses. Reciprocal pathways linking midline limbic structures (cingulate, hypothalamus, hippocampus, amygdala) with widely distributed brainstem, striatal, paralimbic, and neocortical sites are now well-defined
(9,
10), and clear associations with specific motivational, affective, and emotional behaviors in animals have been demonstrated
(3,
4,
11). By extrapolation, these regions are also assumed to be the neural substrates responsible for the systematic integration of exteroceptive and interoceptive inputs required for the combined sensory, cognitive, and autonomic processing at the core of normal and abnormal human emotional experience.
Previous human experiments have generally approached the brain localization of mood and attention in isolation and have rarely addressed mechanisms underlying their interaction
(12,
13). With the use of complementary lesion-deficit and physiological studies
(14–
18), components of attention have been localized to right prefrontal, parietal, and dorsal anterior cingulate regions in both humans and primates. Mood has been mapped to predominantly limbic and paralimbic areas (cingulate, insula) and prefrontal cortex, but with considerable variability
(19–
24). Focal limbic (anterior cingulate), paralimbic (ventral frontal, anterior insula), and neocortical (prefrontal, parietal) abnormalities have also been identified in depression
(25–
29). However, correlations of these regional changes with specific symptoms or relationships between regions have not been fully explored
(30).
To clarify mechanisms mediating these known mood-attention relationships, this study targeted interregional interactions and regional reciprocity in functional brain activity present during both transient and sustained changes in affective state. Using positron emission tomography (PET), we investigated two experimental manipulations of mood: transient sadness provoked in healthy volunteers and treatment-induced resolution of sustained dysphoria in clinically depressed patients. On the basis of the location and direction of changes in neural activity seen in these studies, we propose that the well-recognized concurrence of negative mood and impaired attentional processing in both normal and pathological conditions is mediated by reciprocal interactions between specific limbic and neocortical sites.
METHOD
For experiment 1, healthy subjects were recruited by advertisement and word of mouth. Potential participants were asked to prepare scripts describing two recent sad personal experiences, and these were reviewed with the investigators during the screening interviews. The potential subjects were screened for the presence of medical, neurological, and psychiatric conditions, personal or family history of major affective disorder, and medication use, any of which precluded participation in the study. Semistructured interviews confirmed the absence of major or minor depressive symptoms and evidence of other previously unrecognized psychiatric syndromes or complaints. Potential subjects were then tested on their emotional reactions to their prepared scripts. Of 13 potential subjects so screened, eight right-handed euthymic women (mean age=36 years, SD=6; mean Beck Depression Inventory score=1.75, SD=1 [asymptomatic range=0–9]) were selected for participation on the basis of their ability to predictably provoke an intense sad mood state under laboratory conditions.
For experiment 2, patients who had responded in a 6-week inpatient, placebo-controlled, double-blind PET study of fluoxetine treatment in depression (20 mg/day fixed dose) were identified. All patients met the DSM-IV criteria for a major depressive episode, unipolar type, as diagnosed by two independent psychiatrists (J.A.S. and J.L.T.) and confirmed by means of a structured psychiatric interview (Structured Clinical Interview for DSM-IV
[31]). Patients with cerebrovascular risk factors or a previous stroke, documented head trauma, neurodegenerative disorders, other axis I psychiatric diagnoses, or evidence of global cognitive impairment were excluded, as were patients with psychotic symptoms, alcohol or substance abuse, or previous ECT. Responders were defined as patients who had a minimum 50% decrease in Hamilton Depression Rating Scale score, independent of treatment group (i.e., fluoxetine or placebo). Eight of 15 participating male patients met these criteria (mean age=44 years, SD=8; initial 17-item Hamilton depression scale score=22, SD=3; 6-week score=11, SD=2.5). Four responders received fluoxetine, and four received placebo. Written informed consent was obtained from all subjects, and the experiment was conducted as approved by the University of Texas Health Science Center’s institutional review board.
Experiment 1
Regional cerebral blood flow (rCBF) was measured with the use of [15O]water and PET in two mood states: sad and neutral (euthymic). For each subject there were two trials in the sad condition and four neutral trials (three during rest with eyes closed and one with a neutral-emotion script) in a randomized design. Rehearsed autobiographical scripts were used to provoke the sad (or neutral) mood state. On the basis of prescan practice trials with each subject, the protocol allowed 8–10 minutes to achieve the maximum target mood. Mood state was then maintained, without active rumination on the script, for approximately 2 minutes before tracer injection. Scans were acquired during this state with the subject’s eyes closed. While monitoring of one’s internal mood state (whether sad or neutral) was required for all conditions, the intent of the protocol was to minimize participation of brain regions mediating the cognitive processes involved in generating the emotional state. Subjects were debriefed after completion of each 90-second scan, and mood was allowed to return to a baseline euthymic state before initiation of the next condition, generally after about 5 minutes. Mood state was rated every 2 minutes throughout the session with a visual analog scale and the subject’s self-report. Rest or neutral mood conditions required a score of 6 of a maximum 7 on the calmness scale and a mandatory 0 of 7 on the sadness scale to initiate scan acquisition; sad mood conditions required a minimum score of 6 of 7 on the sadness scale.
Experiment 2
Changes in regional glucose metabolism associated with remission of both dysphoric mood and other syndromal features were measured by means of [18F]fluorodeoxyglucose (FDG) and PET in the selected subset of unipolar depressed patients. Scans were performed before and after 6 weeks of treatment as described above. Scans were acquired with the patients in the resting state, with eyes closed and ears uncovered. The patients were specifically instructed to relax, stay awake, and avoid rumination on any one topic. They were not explicitly instructed to monitor internal mood state. This approach aimed to examine regional effects associated with a sustained shift in overall mood state accompanying remission of illness without any overt acute manipulation of either affective or cognitive state.
General PET Method
All PET scans were acquired on a General Electric Scanditronix 4096 camera (15 parallel slices; 6.5-mm center-to-center interslice distance) with measured attenuation correction (68Ge/68Ga transmission scans) and were reconstructed to a final in-plane resolution of 7.0 mm full width at half maximum. In experiment 1, CBF was measured with a conventional bolus [
15O]water technique (60–65 mCi [
15O]water dose per scan; scan duration=90 seconds)
(32). Glucose metabolism was measured in the resting state with FDG (5 mCi FDG dose; scan duration=20 minutes) following a standard 40-minute FDG uptake period
(33). An anatomical magnetic resonance imaging (MRI) scan of each subject was also acquired for the purposes of spatial transformation of the PET data and parametric image display (Elscint Gyrex 2T-DLX, Haifa, Israel) (three-dimensional gradient/recall acquisition in the steady state sequence: TR=33 msec; TE=12 msec; flip angle=60°; 256×256×127 volume; spatial resolution of 1 mm
3).
PET Data Analysis
All analyses were performed with in-house software and validated methods. Following spatial transformation of PET and MR images into proportional bicommissural coordinate space
(34) relative to the 1988 stereotaxic atlas of Talairach and Tournoux
(35), voxelwise tissue activity of either [
15O]water or FDG was first value-normalized to mean whole brain activity and then scaled to an arbitrary mean of 1000
(32). Value- and spatially normalized images were then trilinearly interpolated, resampled (60 slices; 8-mm
3 voxels), and Gaussian-filtered to a final resolution of 9.9 mm (full width at half maximum) before statistical analysis.
State-related changes in rCBF (experiment 1) and metabolism (experiment 2) were detected in the following manner. A pixel-by-pixel subtraction of the condition pairs was first performed for each trial in each individual. Within-subject differences were then averaged across subjects, creating a grand-mean difference image for each experiment (value for sad memory minus value for rest in healthy subjects; value with treatment minus baseline value in depressed patients). A beta-2 statistic measuring kurtosis of the histogram of the difference image (change distribution curve) was used as an omnibus test to assess overall significance
(36). The beta-2 test was implemented in the MIPS software (Research Imaging Center, San Antonio) in a manner similar to the use of the gamma-2 statistic
(37). The beta-2 improves on the gamma-2 by using a better estimate of the degrees of freedom (i.e., “number of resyls” in the PET images)
(38). The omnibus test was followed by a maxima and minima search to identify local extrema within a search volume measuring 125 mm
3 (39). To facilitate reporting and visualization of these maxima and minima, group mean subtraction images were converted post hoc to statistical parametric images of z scores based on the variance of all local changes within the subtraction images. Locations of concordant focal maxima and minima exceeding a z score magnitude of 1.96 (p≤0.05, two-tailed) are listed in
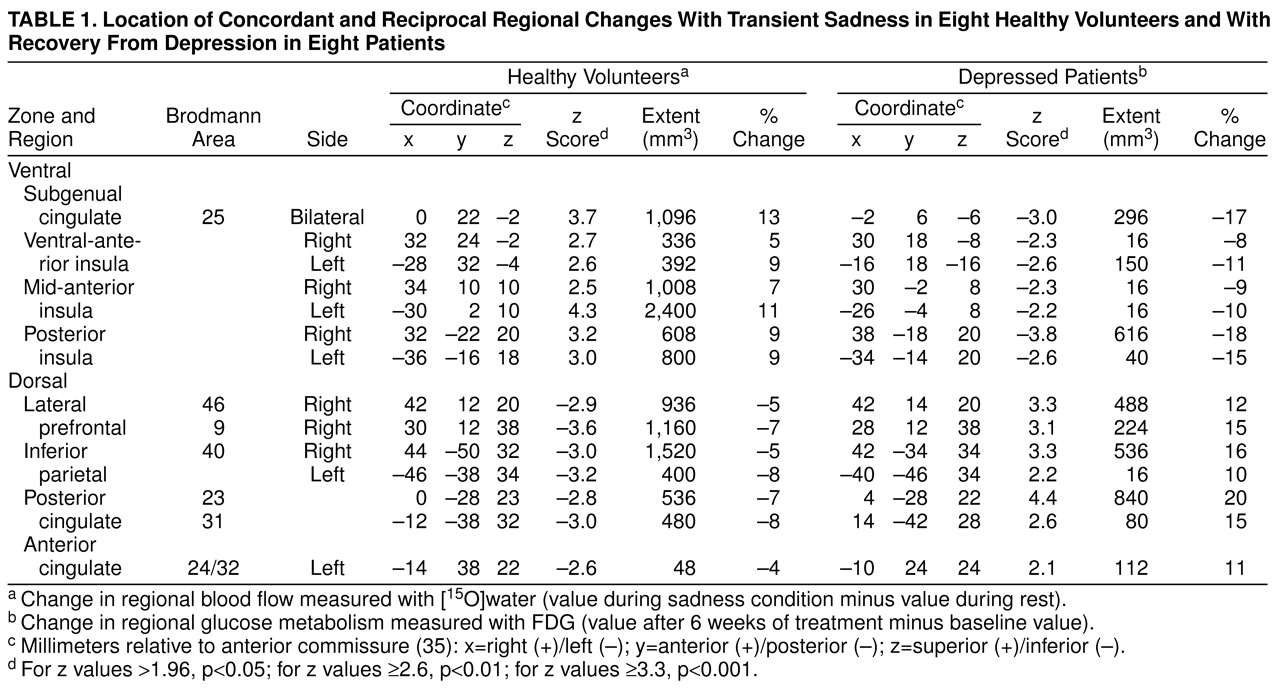
table 1, with the peak voxel of each area described in x, y, and z coordinates in millimeters relative to the anterior commissure. Concordance was defined as a common Brodmann area identifier for the coordinates of the maxima or minima locations in both experiment 1 and experiment 2
(40), as well as evidence of anatomical overlap in the extent of significant activations or deactivations seen with the use of a logical contrast of the two z score maps (
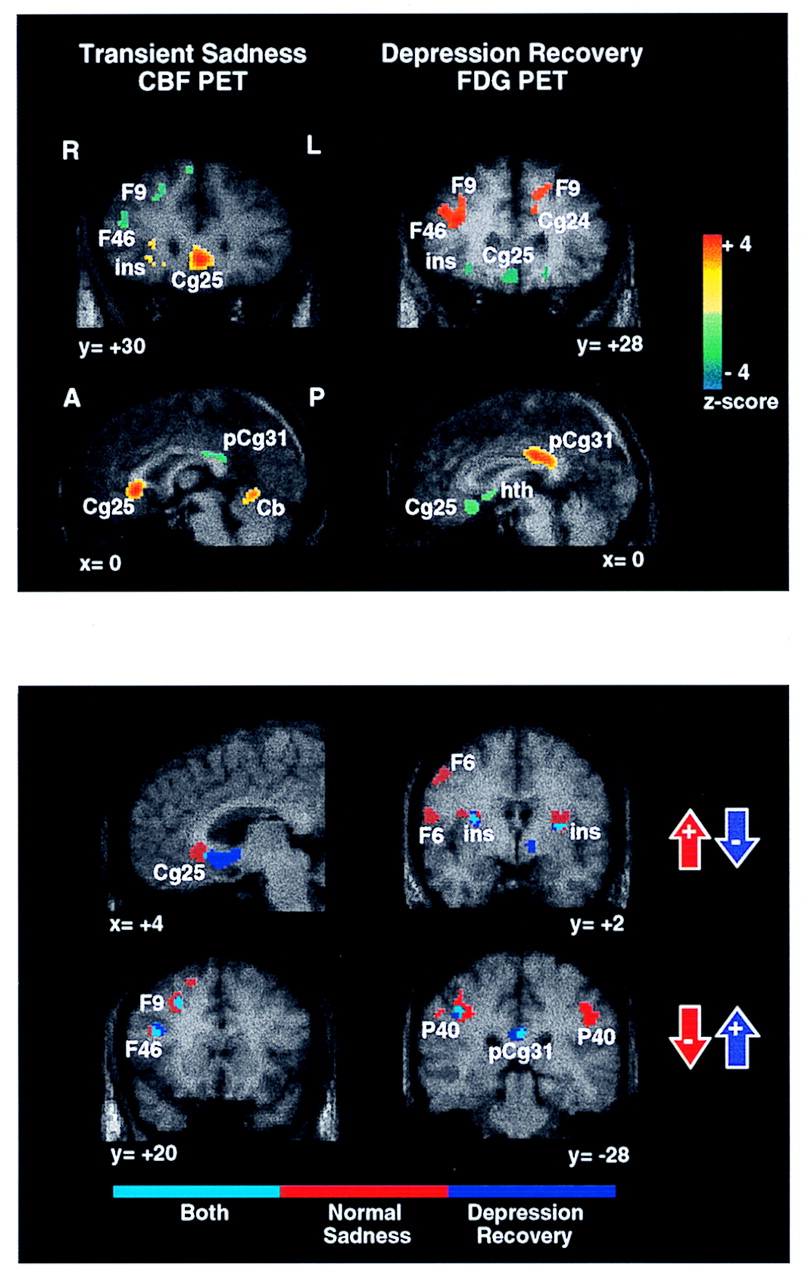
Figure 1, bottom)
(41).
DISCUSSION
This study, using two separate subject populations and two different functional imaging approaches, independently demonstrated reciprocal changes involving nearly identical limbic-paralimbic and neocortical regions with both acute and chronic changes in negative mood state.
In the first and more direct assessment of brain regions mediating negative mood, the induction of transient normal sadness revealed most significantly increases in subgenual cingulate (Brodmann area 25) and decreases in right prefrontal cortex (Brodmann area 9). The increases in subgenual cingulate closely replicate previously reported findings in transient sadness with the use of a variety of provocation methods
(12,
20). In contrast, the observed right prefrontal decreases (as well as the less significant decreases in inferior parietal and dorsal anterior cingulate) are the exact inverse of those seen in published studies where scanning occurred during overt processing of the sad stimuli
(19,
20,
24). Such decreases are nonetheless comparable to those repeatedly described in resting-state studies of patients with clinical depression
(26,
42). Furthermore, these decreases localize to areas previously implicated in both imaging and behavioral studies of attention
(14,
18). In other words, the experience of sadness, as studied here, resulted not only in limbic activation but also in the simultaneous deactivation of cortical regions known to mediate attentional processing, independent of mood state. This observed reciprocal relationship between limbic increases and cortical decreases also appears consistent with changes in environmental awareness described by the subjects in the debriefing interview, as well as the large body of experimental evidence associating negative mood with impaired attention
(8). While performance on attentional tasks was not explicitly monitored during or following the scanning period, performance deficits have been demonstrated in a separate event-related-potential study in which the identical sadness provocation method was used in a different group of subjects
(43).
These reciprocal limbic-cortical and mood-attention relationships were confirmed, although more indirectly, in the second experiment, where resolution of chronic negative mood symptoms accompanying recovery from a chronic depressive episode was examined. The observed right dorsolateral prefrontal increases (also, increases in left prefrontal and dorsal anterior cingulate) are the normalization of a pretreatment frontal hypometabolic pattern characteristic of unipolar depression (relative regional glucose metabolism expressed as value-normalized tissue counts: pretreatment=1185, SD=108; posttreatment=1285, SD=25; healthy subjects=1236, SD=151). These pretreatment abnormalities have been linked to both severity of depression and impairments in psychomotor speed and cognition
(26,
30,
42) and are consistent with the direction of cortical changes seen during acute sadness in experiment 1. The cortical increases accompanying remission generally replicate previous reports of antidepressant treatment effects
(26,
44–
46). In addition, resolution of mood symptoms in the present study was mirrored by significantly improved attentional performance. Simple reaction times were inversely correlated with right prefrontal cortex metabolism (r=–0.47, N=16, p=0.05), consistent with the initial hypothesis that changes in mood and attention are behaviorally linked.
In contrast to the normalization of pretreatment abnormalities seen in cortical regions, the decrease in the ventral limbic region (subgenual cingulate) was not the normalization of an existing abnormality. Rather, this change was suppression of activity below the expected normal level (relative regional glucose metabolism: healthy subjects=1115, SD=100; pretreatment=1119, SD=89; posttreatment=940, SD=126). This decrease in subgenual activity has not been previously described with pharmacological treatment of depression but has been suggested by changes reported with ECT
(47), although anatomical localization of changes with ECT are less precise than those reported here. This relative subgenual cingulate suppression is also consistent with resting state decreases seen in remitted depressed patients on maintenance antidepressant therapy
(48). These pretreatment and remission-related findings—normal subgenual cingulate (Brodmann area 25) metabolism when depressed; subnormal metabolism when well—would appear to contradict a recent report of subgenual cingulate/medial prefrontal hypometabolism and atrophy in acutely depressed unipolar and bipolar patients
(49). This apparent contradiction can be reconciled if the reported hypometabolism is corrected for the described regional atrophy, which would likely result in a metabolic pattern similar to those reported here (i.e., normal or slightly increased)
(50).
A potential confounding factor in considering the interpretation of these data, despite the regional convergence across the two experiments, is the contribution of the neutral control conditions. It has been proposed that task-associated decreases can be caused by suspension of activity present during a passive task or resting state
(51,
52). This does not appear to apply here, since in the transient mood-induction experiment, comparable decreases were seen during both a resting state and a neutral memory condition (i.e., one that more precisely matched the cognitive activity present during the sadness trial)
(53). This theory also would not explain the cortical increases seen in experiment 2. While the debriefing interviews revealed no specific repetitive cognitive thoughts in any of the patients nor subjective differences between the two scans, one might have expected, on the basis of previous reports targeting random episodic thinking, increased uncontrollable intrusive thoughts in the acutely depressed state (scan 1), resulting in a decrease rather than an increase in prefrontal and parietal metabolism with remission of illness
(52).
A second potential confounding element was the presence of tearfulness in six of the eight subjects in experiment 1. While there was inadequate power to assess these effects independently, inspection of single-subject subtractions revealed similar patterns of limbic increases and cortical decreases in subjects who did and did not become tearful. In addition, changes in these same areas were observed with the resolution of negative mood symptoms in the second experiment, even though tearfulness was not observed in any of the patients during either scan.
To recapitulate, concordant changes in nearly identical dorsal cortical and ventral limbic regions were demonstrated in both experiments (
Figure 1, top and bottom). The two areas demonstrating the greatest magnitude of change, subgenual cingulate (Brodmann area 25) and right prefrontal cortex (Brodmann area 9), were also strongly intercorrelated. Using regions of interest (216 mm
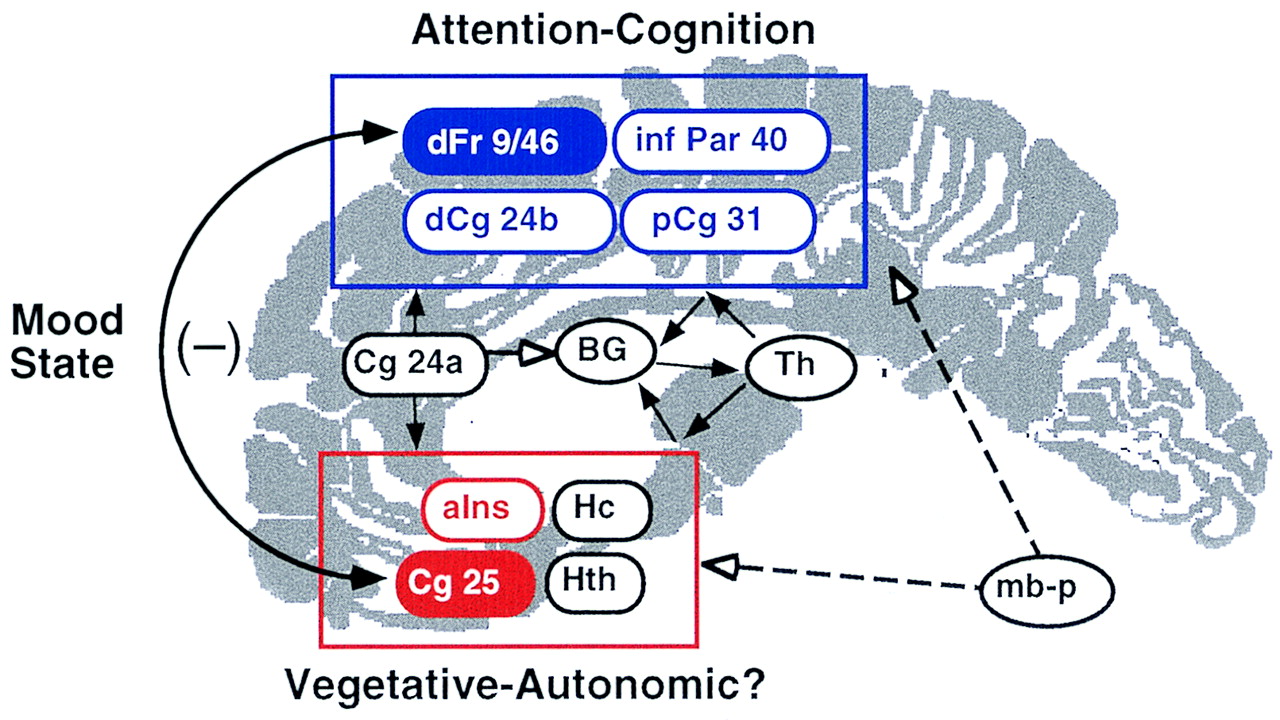
3) centered on the maximal pixel identified in the two subtraction analyses, we found an inverse correlation between the activity in these two regions in both studies (sadness computed for all sadness and resting trials: r=–0.52, N=30, p=0.003; remission computed at baseline, 1 week, and 6 weeks: r=–0.46, N=24, p=0.02). These findings suggest that a reciprocal relationship between ventral limbic and dorsal cortical compartments is maintained through the interaction of these two nodes as mood shifts in either direction (
Figure 2). This statistical relationship further suggests that the negative influence of depressed mood on attention is probably attributable to functional connections between these two brain regions rather than concurrent changes in both sites independently.
The theory that these two regions are functionally linked and mutually inhibitory is supported by a large preclinical animal literature. Direct projections from subgenual cingulate to targets in dorsolateral prefrontal cortex have been delineated, as have bidirectional indirect pathways through multiple limbic and paralimbic nodes, including posterior cingulate, hippocampus, and anterior insula
(4,
9,
10,
54).
The reciprocal nature of the changes observed in these two experiments suggests that treatments for depression targeting either “old” or “new” brain areas in this local circuit should be equally effective—a hypothesis supported by empirical clinical experience and by the finding of comparable regional changes in both drug and placebo responders. Mood symptoms can be ameliorated with cognitive therapy, which implies a “top-down” cortical influence on limbic pathways
(55). Pharmacological treatments are more classically viewed as acting “bottom-up” (or combined bottom-up and top-down), since brainstem nuclei—most notably locus ceruleus and dorsal raphe—are major sites of action of most antidepressants, with secondary effects mediated in remote cortical sites through afferent projections and postsynaptic receptor mechanisms
(56,
57). A more pure bottom-up effect is implicated by the surgical destruction of the subgenual cingulate region and surrounding white matter (the so-called limbic leukotomy or subcaudate tractotomy)
(58), used to treat the most severely treatment-resistant depressed patients, suggesting that the critical change mediating clinical improvement is limbic suppression with secondary cortical release or disinhibition. These clinical observations might indicate that the requisite regional perturbation necessary for remission of disease is neither limbic nor cortical but a reconfiguration of their mutual interactions, whether initiated through top-down (cortical-limbic) or bottom-up (limbic-cortical) interventional strategies
(42). If one assumes that these two approaches are equally viable, the related question of whether 1) suppression of ventral limbic regions allows normalization of dorsal cortical hypometabolism or 2) normalization of cortical activity causes a decrease in ventral limbic regions becomes moot. Clearly, this hypothesis requires examination of additional groups of patients of both genders as well as the focused evaluation of antidepressant medications that are not selective serotonin reuptake inhibitors. Further studies are also needed to delineate the specific behavioral correlates of the other identified regional changes, as well as to clarify how these results may be incorporated into other current theories of depression and mood regulation
(5,
6,
59). Nonetheless, taken together, these data suggest that pathways connecting subgenual cingulate and dorsolateral prefrontal cortex are fundamental to the mediation of mood-attention interactions necessary for maintenance of emotional homeostasis in health and disease.