Marijuana, or
Cannabis sativa, is one of the most commonly used illicit drugs, with over 12 million users in the U.S. alone
(1) . The acute behavioral effects of cannabis include perceptual distortions, paranoia, altered time perception, and occasional hallucinations, along with concomitant disruptions in short-term memory, attention, concept formation, and motor coordination
(2 –
4) . The similarities between these effects and many of the symptoms of schizophrenia have led several researchers to postulate that the cannabinoid system may play a key role in schizophrenia spectrum disorders
(3,
6 ι) .
Andreasen
(10) proposed that the symptom hetereogeneity observed in psychotic disorders (hallucinations, thought disorder, neurological soft signs, etc.) may be understood in terms of a general desynchronization of cortical circuitry. Thus, a possible neurophysiological link between schizophrenia symptoms and cannabis’ neurobehavioral effects may involve alterations in neural synchronization. Cellular studies and computational models have suggested that rhythmic synchronization across neuronal assembles in the beta (15–30 Hz) and gamma (30–80 Hz) range plays an important role in the integration and binding of perceptual features, associative learning processes, and conscious awareness
(11,
12) . In humans, neural synchronization can be assessed noninvasively by entrainment of the EEG to periodic sensory stimuli (e.g., auditory click trains at specific frequencies). Because the steady-state EEG waveform entrains to the frequency and phase of the presented stimulus, it serves as an indicator of the functional state of the neural circuits supporting synchronization
(13,
14) .
Direct evidence of altered neural synchronization using the evoked entrainment paradigm has been shown in schizophrenia patients. Patients demonstrate reductions in both spectral power and phase synchrony of steady-state visual and auditory evoked potentials. In the visual modality, this reduction is most pronounced in the alpha (6–13 Hz)
(15,
16) and beta-range frequencies (17–30 Hz)
(17,
18) . However, such dysregulation is more marked in the auditory modality, particularly at 40 Hz of stimulation
(17,
19 β1) . In addition to disruptions in synchrony using evoked steady-state responses, several studies have demonstrated schizophrenia deficits in transient induced synchronization (gamma band) to gestalt images
(22) and during auditory discrimination paradigms
(23,
24) . Thus, the ability of neural networks to support synchronous activity appear to be compromised in psychotic patients.
The neurochemical correlates of neural synchrony have been shown to be modulated by γ-aminobutyric acid (GABA)-ergic interneurons and metabotropic and ionotropic glutamate receptors
(12) . Another putative mechanism may relate to the neuromodulatory actions of endocannabinoids. For example, it is now known that central cannabinoid (CB1) receptors are colocalized with GABA interneurons
(25,
26) . More directly, Hajos et al.
(27) demonstrated that administration of the highly potent cannabinoid agonist CP55,940 markedly reduced the power of 40-Hz oscillations elicited in hippocampal slices by kainate. Thus, cannabinoid activity, either through endogenous or exogenous agonist administration, may exert neuromodulatory control of GABA-ergic and glutamatergic-dependent network oscillations. To date, no studies have sought to ascertain whether cannabis use produces alterations in neural synchronization in humans.
Method
Subjects
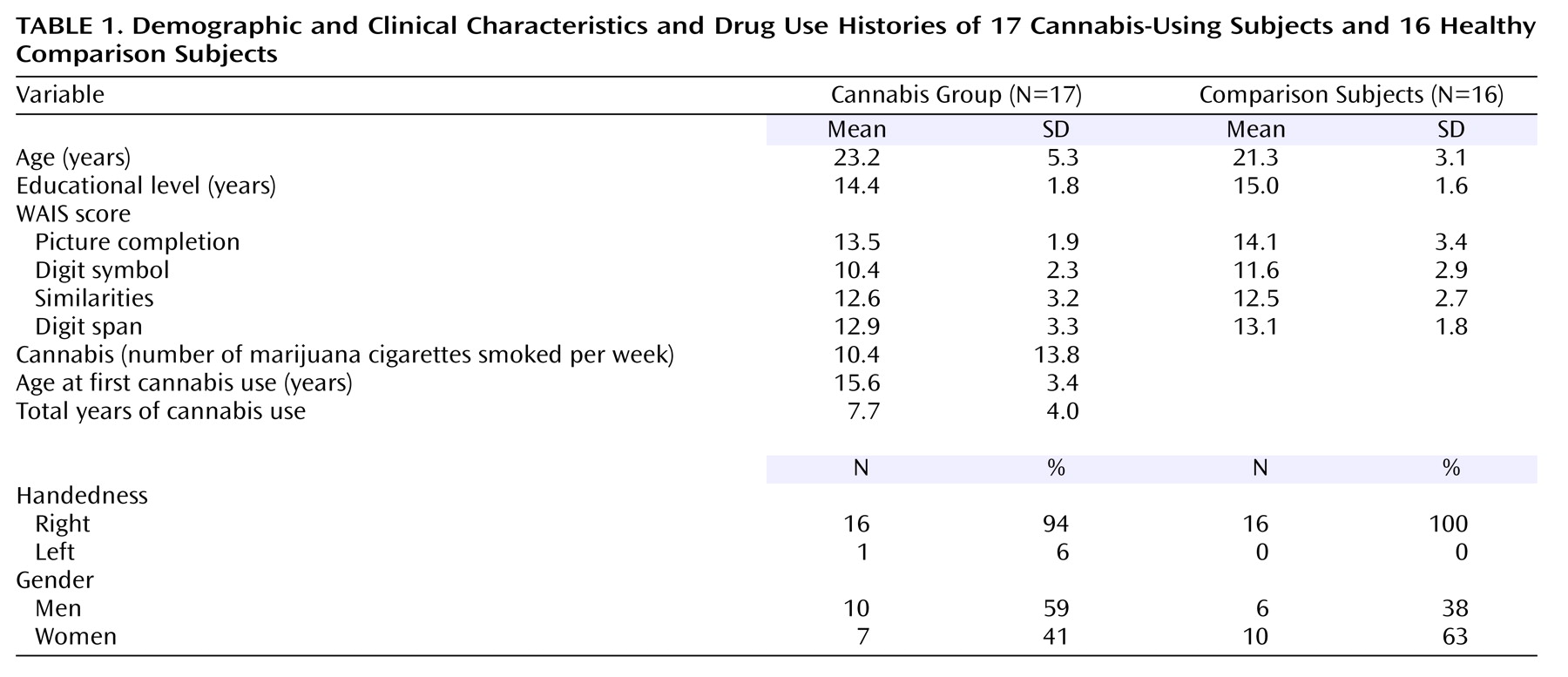
Current cannabis users (N=17) and healthy drug-naive comparison subjects (N=16) were assessed. The subjects were recruited from the local university community and paid for their participation in the study; written informed consent was obtained from each.
Table 1 shows participant demographic, clinical, and cannabis use characteristics. There were no significant differences between the groups in age (F=1.71, df=1, 31, p=0.20) or years of education (F=1.19, df=1, 31, p=0.28).
The inclusion criteria were as follows:
1. For the cannabis group: current cannabis consumption (marijuana cigarettes smoked) at the rate of at least once per week (during the past month), and no other illicit substance use during the past 6 months.
2. For the drug-naive comparison subjects: no history of illicit substance use.
3. For all subjects: ages 18–35, completion of high school education, no history of DSM-IV axis I or II disorders other than cannabis dependence, no history of cardiovascular disease, hearing problems, neurological disease, learning disability, or head injury resulting in loss of consciousness.
In addition, subjects were excluded if they reported consumption of more than two alcoholic drinks per day (one per day for women). The cannabis group’s drug-use inclusion criteria (cannabis use at least once per week, 24-hour abstinence) were chosen to eliminate acute cannabis effects while retaining neurophysiological effects from residual cannabinoids. Previous work has shown that several areas of neuropsychological performance remain affected up to 7 days after cessation of cannabis use (possibly because of its long half-life and the fat solubility of tetrahydrocannabinol [THC]), and this heuristic was used in the current study
(28,
29) . Finally, it should be noted that although none of the cannabis-using subjects had used other substances during the past 6 months, several participants (10 of 17) reported polydrug use in the past (cocaine, amphetamines, LSD, and 3,4-methylenedioxymethamphetamine [MDMA or Ecstasy]). Comparisons of the pure cannabis users and the past polydrug cannabis users on each of the measures described in the following paragraphs yielded no significant differences.
Clinical Interviews and Questionnaires
The following psychometric questionnaires and clinical interviews were administered to all subjects before EEG recording: the Structured Clinical Interview for DSM-IV Axis I and II disorders (SCID I and SCID II), a substance use questionnaire, the Schizotypal Personality Questionnaire
(30), the Perceptual Aberration Scale
(31), the Dissociative Experiences Scale (DES)
(32), and subscales of the WAIS-III (picture completion, digit symbol, similarities, and digit span).
Using the time-line follow-back procedure, measures of frequency, quantity, and density of cannabis consumption were determined, with the clinical interview for the past 6 months, then for 1 month before the test session. The subjects were instructed to consider each day of the week and indicate, for an average week, how much they consumed per drug-use occasion over the past 6 months and then for the month before testing. Recency or density of last use was assessed using the past-month section of the interview.
Stimuli and EEG Recording Procedure
Auditory stimuli consisted of click trains (square waves) presented at three different frequencies in each of three blocks (20, 30, and 40 Hz at 80 dB sound pressure level). Each block contained 100 trials of each frequency presented for 500 msec each (interstimulus interval of 1000 msec) and were presented through foam insert earphones (Etymotic Research, Elk Grove Village, Ill.). The order of the blocks was randomly assigned across subjects. The subjects were tested in a sound-attenuated room and asked to relax with eyes open and listen to the click trains while sitting in an upright chair with a headrest during the recording.
The EEG was recorded continuously (band pass 0.1–100 Hz; sampling rate 1000 Hz) from the scalp with a 12-channel electrode cap with a nose reference. Electrode impedances were maintained below 10 kΩ. The recorded EEG was segmented into epochs consisting of the 500 msec during stimulus presentation, with any epoch containing a voltage greater than 100 μV excluded. Ocular movement artifact correction was applied with the algorithm of Gratton et al.
(33), and averages were computed for each frequency block with commercially available software (Vision Analyzer, Brain Products GmbH, Gilching, Germany).
EEG Data Analysis
Spectral Power
A power spectrum was obtained from the averaged EEGs for each condition and subject using a Fast Fourier Transform (Vision Analyzer, Brain Products). The Fast Fourier Transform was performed on the time interval during stimulus presentation (500 msec). The average signal power from all subjects in each group for 12 channels (three frontal, three central, one parietal, two temporal, and three occipital regions: F7, F8, Fz, C3, C4, Cz, T4, T6, Pz, O1, O2, and Oz) at each frequency of stimulation was plotted on a scalp map using interpolated data to examine the scalp distribution of the responses. Confirming previous research on auditory steady-state evoked potentials
(17,
19 β1), the signals showed local maxima in frontal-central regions, and all statistical analyses were conducted on data from electrodes F7, F8, Fz, C3, C4, and Cz.
Signal-to-noise ratio
The power of the averaged EEG response at the frequency of stimulation (averaged trial power) was used as the signal value and compared to mean single trial power. Single trial power was obtained by calculating the mean power at the stimulus frequency across all trials. Noise was then computed as the difference between the mean of the single trial power for the frequency of stimulation minus the signal (averaged trial power).
Noise=single trial power – averaged trial power
Signal-to-noise ratio=averaged trial power/(single trial power – averaged trial power)
This formula takes the power derived from averaged trial data as the signal, and its difference from power calculated from single trials as noise. This measure of the signal-to-noise ratio has been shown to be affected in schizophrenia for event-related potentials to auditory stimuli during a tone discrimination task
(34,
35) .
Statistical Analysis
Power and signal-to-noise ratio at the frequencies of stimulation were measured for each condition in which the entrainment power was largest (frontal-central electrodes). The log 10 -transformed power and signal-to-noise ratio values were used for each statistical analysis. A repeated-measures analysis of variance (ANOVA) was used to evaluate the between-subject factor of group (two) and the within-subject factor of electrode (six) on the power and signal-to-noise ratios at each stimulation frequency. A one-way ANOVA was used to compare scores on the various questionnaires between groups. In order to examine possible relationships between variables, Pearson correlation coefficients were used. A criterion of p<0.05 was used throughout to determine statistical significance, and all tests were two-tailed.
Results
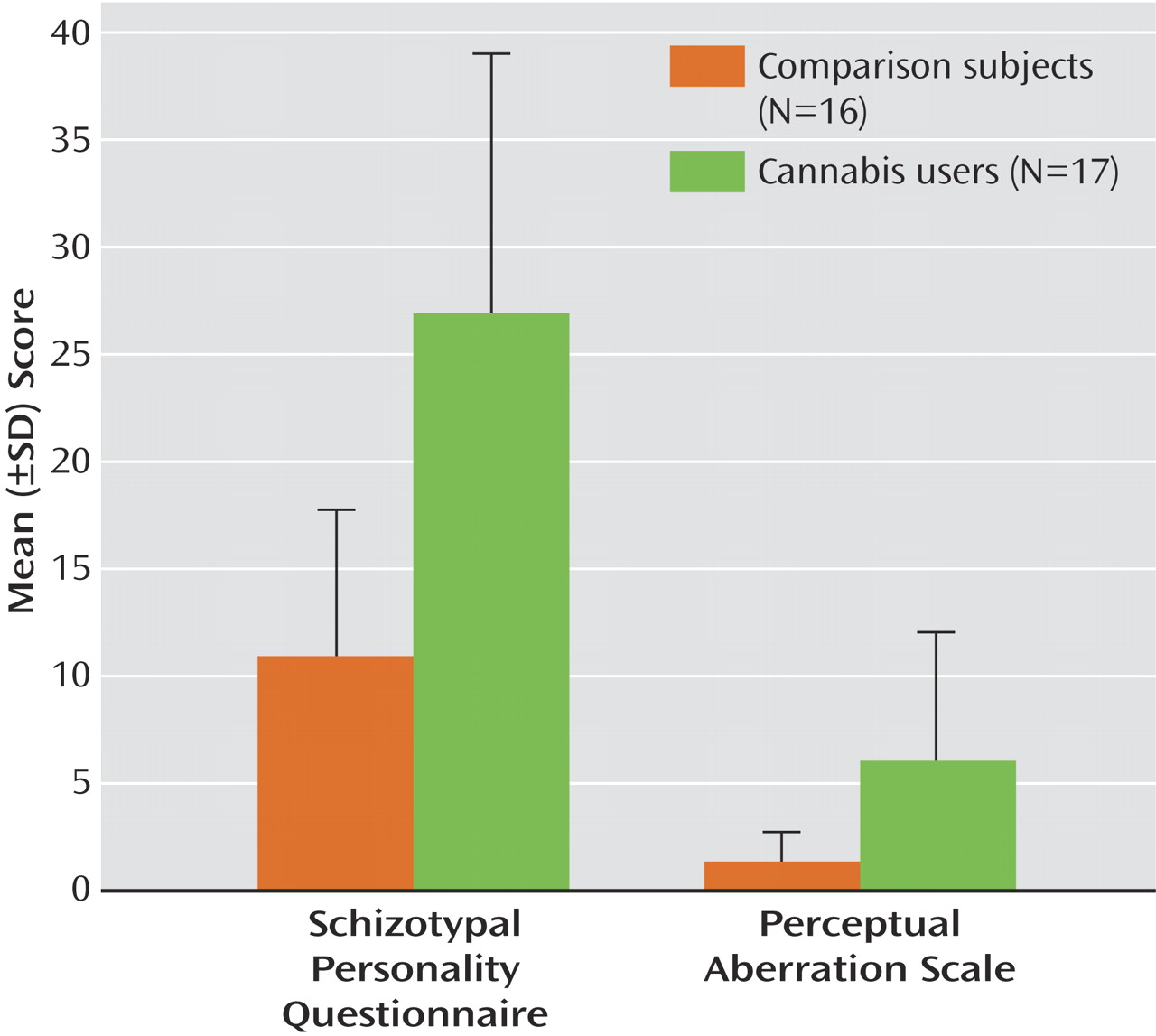
An ANOVA revealed that cannabis users had higher scores on the Schizotypal Personality Questionnaire (F=21.8, df=1, 31, p<0.0001) and the Perceptual Aberration Scale (F=8.9, df=1, 31, p<0.005) (
Figure 1 ). No differences were found for DES or WAIS-III scores. The lack of differences between the groups on WAIS scores indicates that any alterations in neural synchrony are not associated with generalized cognitive or sensory deficits.
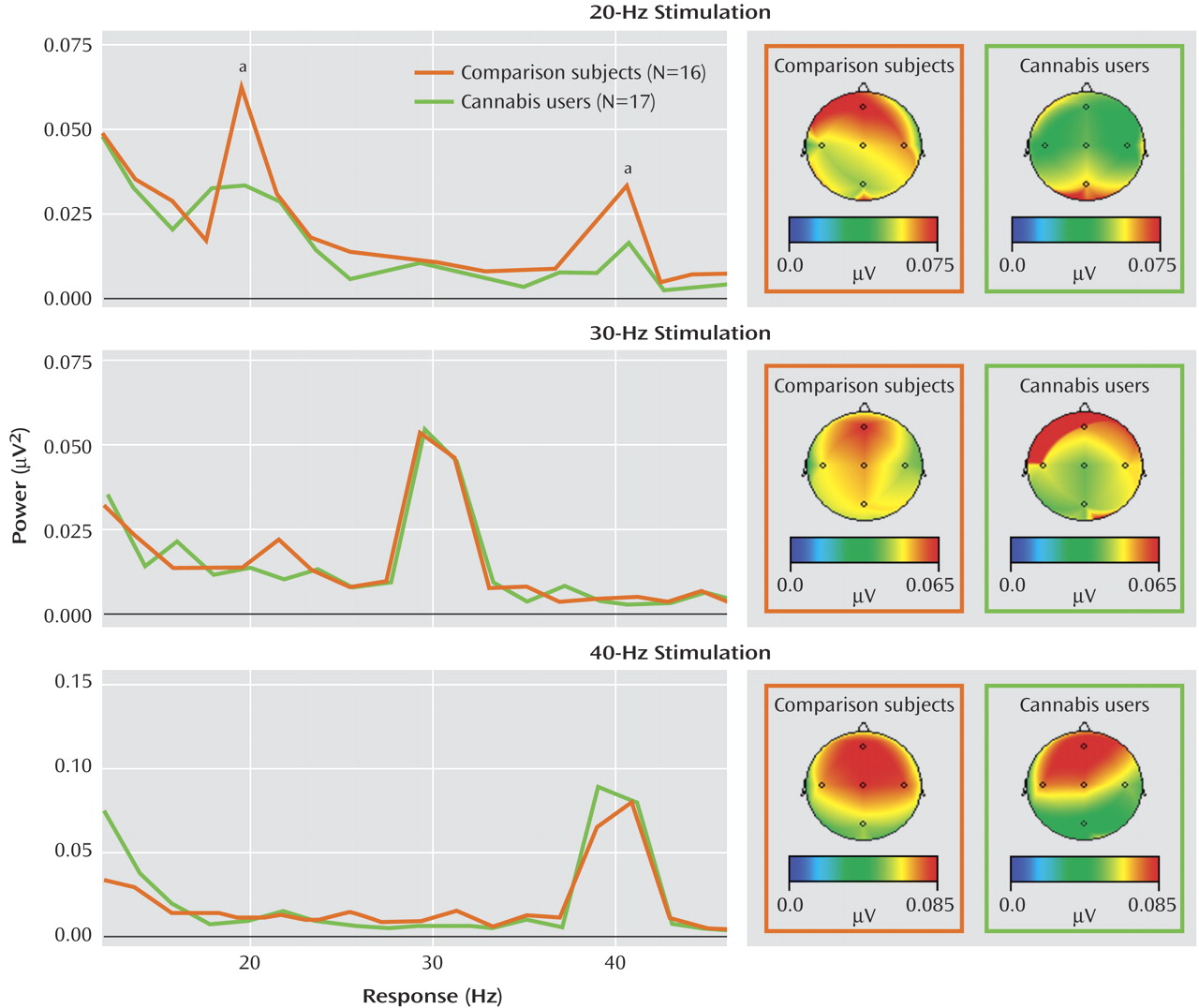
In the auditory entrainment protocol, subjects in both groups entrained to all three stimulus frequencies, which can be seen in the graphs of spectral power taken from electrode Fz (
Figure 2 ). The 20-Hz stimulation also elicited a strong 40-Hz harmonic response, which can be seen in the upper panel of
Figure 2 . A repeated-measures ANOVA examining the effect of group on 20 Hz power across all frontal and central electrodes revealed a main effect of group, indicating that cannabis-using subjects demonstrated significantly less entrainment power during 20-Hz stimulation (F=5.23, df=1, 31, p<0.03). A main effect of group was also observed in the concomitant 40-Hz harmonic response (F=4.55, 1, 31, p<0.05). A significant group-by-electrode interaction was also observed in the 20-Hz condition (F=3.58, df=5, 27, p<0.02). Individual AVOVAs on power at each electrode revealed that although the cannabis-using subjects had decreased 20 Hz power across all frontal-central electrodes, the group differences were significant at C4 (F=5.3, df=1, 31, p<0.03), F7 (F=4.7, df=1, 31, p<0.04), and Fz (F=4.1, df=1, 31, p<0.05). Both groups exhibited the largest 20-Hz response at Fz, and the decreased entrainment in the cannabis-using group in relation to comparison subjects at Fz can be seen in the upper panel of
Figure 2 . The ANOVAs revealed no group differences in the 30- or 40-Hz stimulation conditions.
For signal-to-noise ratios, a significant main effect of group was observed in the 20-Hz condition, indicating that subjects with cannabis had decreased signal-to-noise ratios at 20 Hz (F=5.86, df=1, 31, p<0.03). A significant group-by-electrode interaction was also observed for signal-to-noise ratio at 20 Hz (F=3.1, df=5, 27, p<0.03). Individual ANOVAs indicated that cannabis-using subjects had decreased signal-to-noise ratios across all frontal-central electrodes, with significant group differences at C4 (F=7.20, 1, 31, p<0.01), and F7 (F=6.90, df=1, 31, p<0.01) (Fz and C3 were also lower but were below the level of significance). However, no differences were observed between the groups in noise power for 20 Hz (F=0.47, df=6, 26, p=0.82). This pattern indicates that the group differences in spectral power at 20-Hz stimulation were not due to greater system noise but may be the result of lower signal strength. No differences in the signal-to-noise ratios were found for 30 or 40 Hz of stimulation or in the 40-Hz harmonic during the 20-Hz condition.
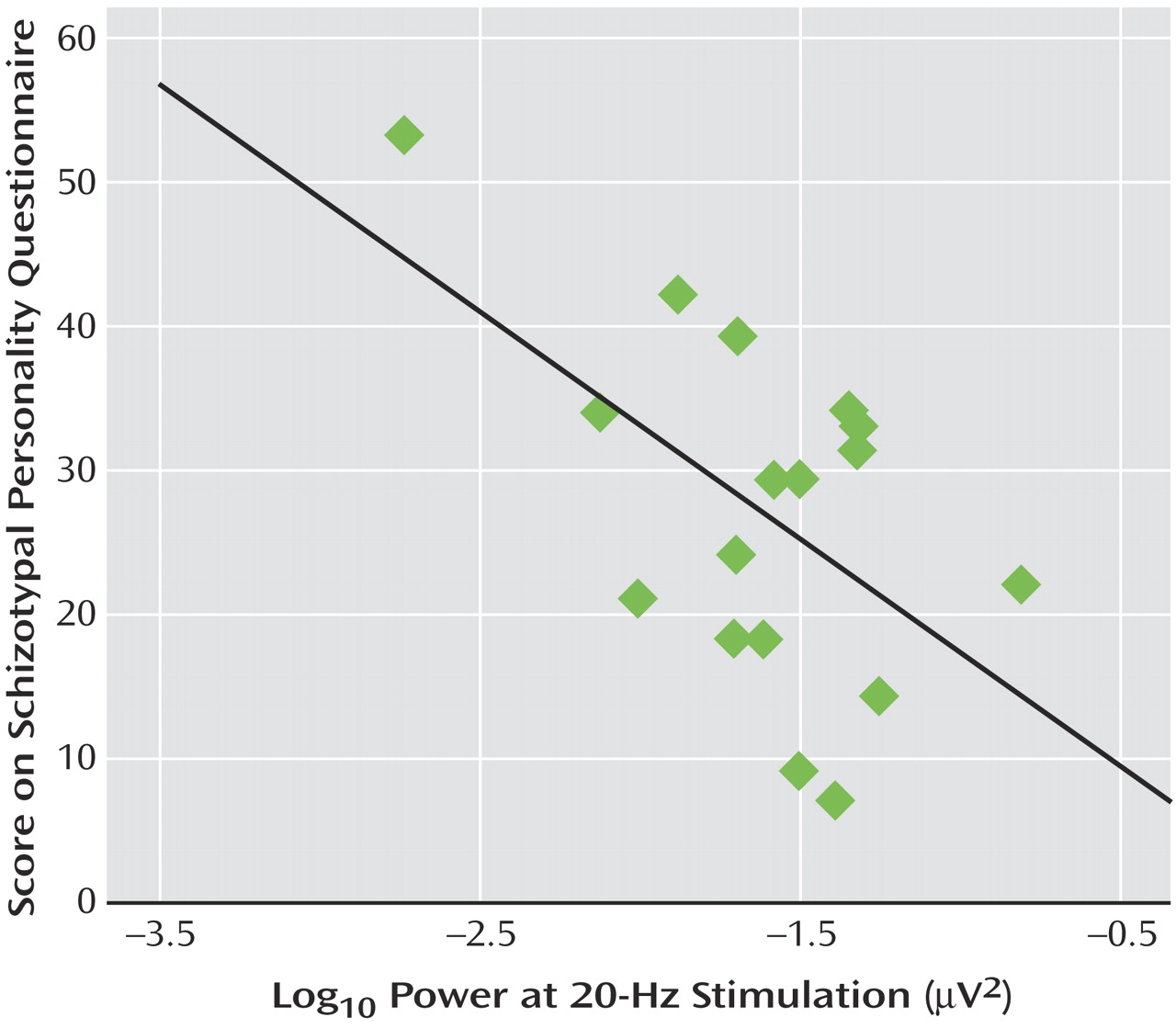
Correlational analyses revealed that within the cannabis group, a significant negative association was observed between 20 Hz power at Fz and Schizotypal Personality Questionnaire scores (r=–0.55, p<0.02). Thus, individuals within the cannabis-using group with the highest schizotypy demonstrated the lowest power at 20 Hz (
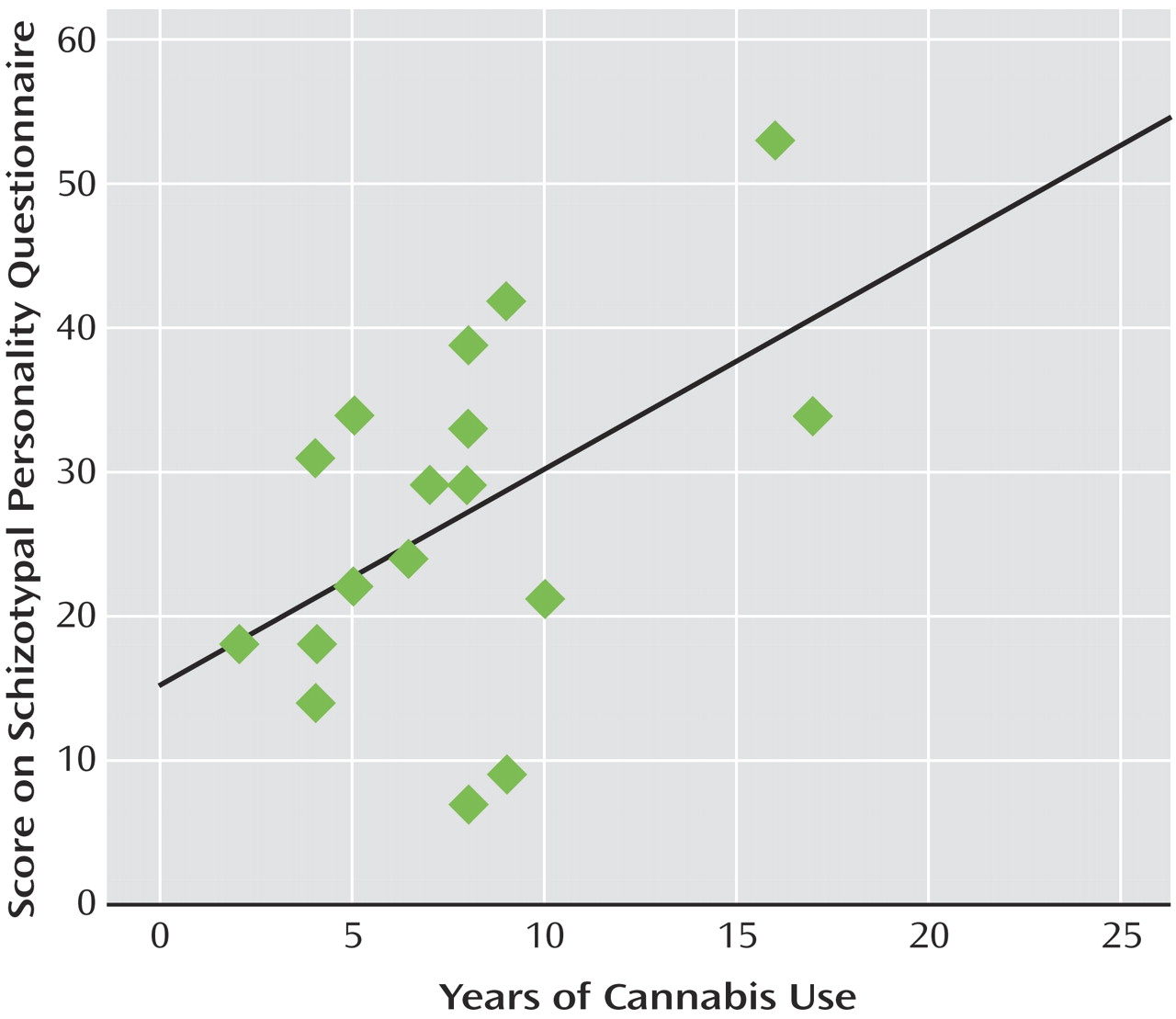
Figure 3 ). This correlation was not apparent for the other frequencies of stimulation or within the comparison group. Finally, within the cannabis group, a significant positive correlation was observed between total years of cannabis use and Schizotypal Personality Disorder scores (r=0.49, p<0.05) (
Figure 4 ).
Discussion
The current study demonstrated that cannabis users exhibited altered neural synchronization as shown by reduced 20 Hz EEG power and signal-to-noise ratio to auditory stimulation. No differences were found between the groups in noise power, indicating that the decreased 20 Hz power may be due to decreased signal strength of oscillating circuits and not the increased noise stemming from neural background activity. Increased perceptual aberration and schizotypy scores were also observed, and the increased schizotypy was positively correlated with total years of cannabis use. In addition, within the cannabis group, schizotypy scores were negatively correlated with 20 Hz power, indicating that cannabis-using individuals scoring higher in schizotypy demonstrated larger deficits in neural synchronization at 20 Hz.
The observed association between cannabis use and schizotypy is consistent with previous research demonstrating increased schizotypy in otherwise healthy cannabis users
(3,
36 γ9) . Although it is difficult to determine the direction of causality of this association without longitudinal studies, previous work has shown that past cannabis users who were drug-free for at least 6 months showed no differences in schizotypy in relation to the comparison subjects
(3) . Furthermore, in the recent study by D’Souza et al.
(9), it has been shown that direct intravenous cannabinoid administration (Δ-9-THC) in healthy subjects can induce acute perceptual aberrations, memory or attentional impairments, and psychotomimetic effects that resemble the symptoms of schizophrenia. Thus, it appears that cannabis use mimics many of the behavioral symptoms observed in schizophrenia and may constitute an independent risk factor for the development of psychosis in vulnerable individuals
(40 –
46) . Indeed, it has recently been shown that individuals with a predisposition toward psychosis who later consume cannabis are at a greater risk for psychotic symptoms
(47), suggesting a possible gene-by-environment interaction. Toward this end, Caspi et al.
(48) showed that a functional polymorphism in the catechol-
O -methyltransferase (COMT) gene, which is involved in synaptic dopamine metabolism, interacts with adolescent cannabis use to predict adult psychosis. Given that there is tight coupling of the endocannabinoid and dopamine systems
(49), it appears possible that genetic anomalies leading to altered dopamine activity may interact with early cannabis exposure to produce overt psychosis.
The present study is the first to our knowledge to demonstrate decreased auditory steady-state responses in cannabis users. Decreases in this response using similar EEG paradigms have been shown in patients with schizophrenia
(17,
20,
21) . However, the reductions in EEG power in schizophrenia primarily occur in the gamma-range (~40 Hz). It is unclear why the observed reduction in power was confined to the lower frequency of 20 Hz, although it should be noted that the 40-Hz harmonic of 20 Hz was also significantly lower in cannabis users. One possibility is that cannabis consumption modulates beta-range neural synchronization. Computational models suggest that gamma rhythms may represent synchronous activity in local neural circuits, whereas beta rhythms represent synchronization over larger distances in the brain
(50) . Such a role of beta activity has been shown in human EEGs during multimodal object processing in which increased coherence at 12–20 Hz between parietal and temporal electrodes was observed during stimulus processing
(51) . Thus, there may be dissociation between the types of synchronization abnormalities in cannabis use versus full-blown schizophrenia. Although it is purely speculative, it is possible that this differing pattern is due to the fact that the psychotomimetic effects of cannabinoids are subthreshold and may initially affect only beta synchrony. As several studies have also demonstrated that gamma and beta rhythms can occur simultaneously
(52,
53), it is clear that the relationship between gamma and beta synchronization is complex, and future work is needed to clarify the differing patterns of abnormal steady-state responses in cannabis-using and schizophrenia populations.
Concerning the mechanism of cannabis’ effect on neural synchronization, several possibilities exist. The first relates to cerebellar modulation of neural synchrony. The cerebellum is involved in a multitude of nonmotor functions, such as cognition (executive function and attention) and neural timing
(54,
55) . Recently, it has been shown that periodic stimulation in the gamma range causes an increase in cerebellar activity in both auditory and visual steady-state paradigms, as assessed with positron emission tomography
(56,
57) . Because several studies have shown that cannabinoid-dependent timing defects are related to cerebellar activity, cannabis use may cause disruptions in neural synchrony through alterations in neural events in the cerebellum
(58,
59) . These data, along with the fact that cerebellar neurons are replete with GABA-ergic and cannabinoid neurons
(60,
61), both of which are implicated in neural synchronization
(12,
27), provide indirect support that cannabis consumption may modulate synchrony with the cerebellum.
Another possible mechanism of cannabis’ effect on neural synchronization relates to the possible role of endogenous cannabinoids in GABA-ergic network oscillations. In the hippocampus, CB1 receptors are located primarily on cholecystokinin-containing GABA-ergic interneurons
(25 –
27) . Activation of these presynaptic CB1 receptors reduces GABA release by interneurons
(25,
62), which, in turn, could disrupt the synchronization of pyramidal cell activity. The reduced power of network oscillations by cannabinoid administration demonstrated by Hajos et al.
(27) provides initial support for this idea. Such cannabis-modulated desynchronization could thereby interfere with associative functions, disrupt normal gating mechanisms, and eventually lead to psychotic or altered perceptual experiences.
Several issues related to the conclusions of the present study need to be addressed. Levels of cannabis use were determined through self-reports. Hence, inaccuracies in estimated drug use patterns, frequency, and time of last use are possible. However, previous work has shown that self-reports of cannabis use tend to be fairly reliable
(63,
64) . Additionally, because subjects were tested after 24 hour of abstinence, it is unclear whether the observed effects were due to cannabis withdrawal
(65) or residual cannabinoids
(28) . Finally, several of the cannabis subjects reported a past history of use of other illicit substances, which could potentially confound the current results. Each of these concerns could be negated by future studies using both urinary or plasma levels of THC metabolites and assessment of EEGs during direct cannabinoid administration.
A second issue relates to the known attentional deficits observed in cannabis use
(3) . It is possible that the decreased power observed at 20 Hz is mediated by cannabis-induced attentional dysregulation. However, if attentional effects were responsible for the decreased synchronization, then similar patterns would be expected for the other frequencies of stimulation (30 and 40 Hz). In addition, unlike visual steady-state paradigms, there are inconsistent data regarding whether power in the auditory modality is affected by selective attention
(66,
67) . Future studies seeking to concomitantly assess attention and neural synchrony in relation to cannabinoids could address these issues.
Finally, assessments of induced gamma or beta synchronization during object recognition, motion perception, or discrimination tasks are necessary because these paradigms have higher ecological validity and probe processes of perceptual integration that are known to be aberrant in cannabis use. Such studies would serve to elucidate the neurophysiological effects of cannabis on the brain, along with further delineating the relationship between the cannabinoid system and processes known to be affected in schizophrenia.