Working memory enables us to temporarily hold and manipulate information, and it underlies higher-order thinking, language, and goal-directed behavior
(1) . Impairment of working memory is an important component of the cognitive dysfunction seen in schizophrenia
(2 –
4) . Functional magnetic resonance imaging (fMRI) experiments
(5,
6), complemented by primate single-neuron recordings and neurocomputational models
(7), suggest that in healthy persons, higher-order working memory executive processes (e.g., manipulation of information versus simple maintenance) preferentially engage dorsal (Brodmann’s areas 9 and 46) relative to ventral (Brodmann’s areas 44, 45, and 47) regions of the prefrontal cortex. Related work also suggests that the prefrontal cortex is hierarchically organized, with higher-order processes in the dorsal prefrontal cortex exerting control over more ventral regions that are responsible for simpler processes
(8,
9), such as rehearsal of information within working memory
(10,
11) .
Few investigations have explicitly examined the functional hierarchy of distinct dorsal and ventral regions in the prefrontal cortex in patients with schizophrenia, although schizophrenia patients have been found to be selectively more impaired on behavioral working memory tasks that have higher executive demands
(12,
13), where dysfunction in the dorsal prefrontal cortex may be a key factor. Although a number of functional imaging experiments support the presence of dorsal prefrontal cortex dysfunction in schizophrenia
(14 –
19), studies have variously reported increases and decreases in activation of the prefrontal cortex, depending on the specific task paradigm used, and illness-related differences in behavioral performance and capacity constraints of task load
(15,
20 β2) . Moreover, it has been suggested that ventral regions contribute significantly to observed hyperactivity in the prefrontal cortex in some patients and comparison subjects matched on working memory performance
(23,
24) .
In this study we sought to further examine how dorsal-ventral prefrontal functional organization might be altered in schizophrenia, independent of performance deficits. In order to examine higher-order working memory executive processing and dorsal prefrontal cortex function, we focused on the differential activation between 1-back and 2-back tasks, reflecting incremental working memory load (number of target items stored) and information updating (the executive process of continuous selection and updating of target items)
(25) . We studied patients and healthy comparison subjects with similar performance indices and analyzed subgroups defined in terms of memory performance: high-performing comparison subjects, high-performing patients, low-performing comparison subjects, and low-performing patients. Although both ventral and dorsal regions are commonly coactivated in the distributed working memory network, we expected the high-performing comparison subjects to adapt to increased working memory executive load predominantly via activation of the dorsal prefrontal cortex. In patients, greater activation in the ventral prefrontal cortex might occur in addition to or in lieu of activation elsewhere in the prefrontal cortex and could reflect compensatory processes if behavioral performance is maintained. In other words, if patients with schizophrenia have a primary defect in dorsal prefrontal cortex processing, they may resort to cognitive strategies that preferentially engage ventral circuitry as a means of maintaining performance. While this might be a less efficient strategy, at least when manipulation of information becomes more critical, it would be consistent with patients’ also having diminished working memory capacity
(15) .
To examine illness effects on the dorsal and ventral prefrontal cortex, we also analyzed functional connectivity between prefrontal cortex regions and the posterior parietal cortex. The prefrontal cortex-posterior parietal cortex coupling during working memory tasks has long been implicated in human and primate studies of functional and structural anatomy
(8,
26,
27) . We expected to find that schizophrenia patients would have relatively lower functional connectivity between the dorsal prefrontal cortex and posterior parietal cortex, reflecting loss of its specialized role in the working memory network, as would be consistent with recent positron emission tomography (PET) findings
(24) . Conversely, patients would have greater functional connectivity between the ventral prefrontal cortex and posterior parietal cortex compared with healthy comparison subjects, if indeed the ventral activation was compensatory.
Method
We initially studied 24 patients with schizophrenia and 26 healthy comparison subjects, all right-handed as assessed by the Edinburgh Inventory
(28) . Patients were recruited from the Clinical Brain Disorders Branch Sibling Study (NIH Protocol 95-M-6150). Comparison subjects were recruited from the National Institutes of Health Clinical Research Volunteer Program. The study was approved by the institutional review board of the Intramural Program of the National Institute of Mental Health. All study subjects gave written consent before participation. Ten subjects in this study were included in a previous report
(17) .
All participants were assessed with a Structured Clinical Interview for DSM-IV, a neurological examination, a battery of neuropsychological tests, an EEG, and a screening MRI examination. Exclusion criteria included an inability to give informed consent, IQ below 70, a history of substance abuse within the past 6 months, a history of significant neurological illness, and any focal abnormalities on EEG or MRI. All schizophrenia patients were on a stable regimen of antipsychotic medication.
Acquisition of N-back fMRI data was done largely as previously described
(17) . Briefly, the N-back task consisted of continual presentation of visual stimuli in which every number was both a probe and a target (i.e., 100% target). The numbers 1–4 appeared randomly every 1.8 sec for 500 msec at set locations at the points of a diamond-shaped box. Subjects were to recall the stimulus seen N previously by means of a fiber-optic response box with buttons arrayed in the same configuration as the stimuli presented on the screen. The task was presented as two counterbalanced runs in which 30-sec epochs of 0-back alternated with either 1-back or 2-back.
Whole brain blood-oxygen-level-dependent fMRI data were collected on a 3-T GE scanner (General Electric Systems, Milwaukee) with a GE-EPI pulse sequence acquisition of 24 contiguous slices (echo time=30 msec, repetition time=2 seconds, flip angle=90°, field of view=24 cm, matrix=64×64, voxel dimensions=3.75×3.75×6 mm). All fMRI data were processed and spatially normalized to a common stereotaxic space (Montreal Neurologic Institute template), analyzed with SPM99 software (Wellcome Department of Imaging Neuroscience, London, http://www.fil.ion.ucl.ac.uk/spm), and individually examined for motion artifacts as described previously
(15) .
Because activation of the prefrontal cortex has been shown to be sensitive to behavioral performance differences
(15,
20), we matched patients and comparison subjects for 1-back and 2-back performance. We excluded seven patients whose performance was more than two standard deviations below the mean of the comparison subjects and two patients because of movement artifacts during scanning. The patient and comparison groups were each divided into two subgroups based on median performance accuracy at 2-back, thus forming a cohort of eight high-performing patients and 14 high-performing comparison subjects, and another cohort of seven low-performing patients and 12 low-performing comparison subjects (
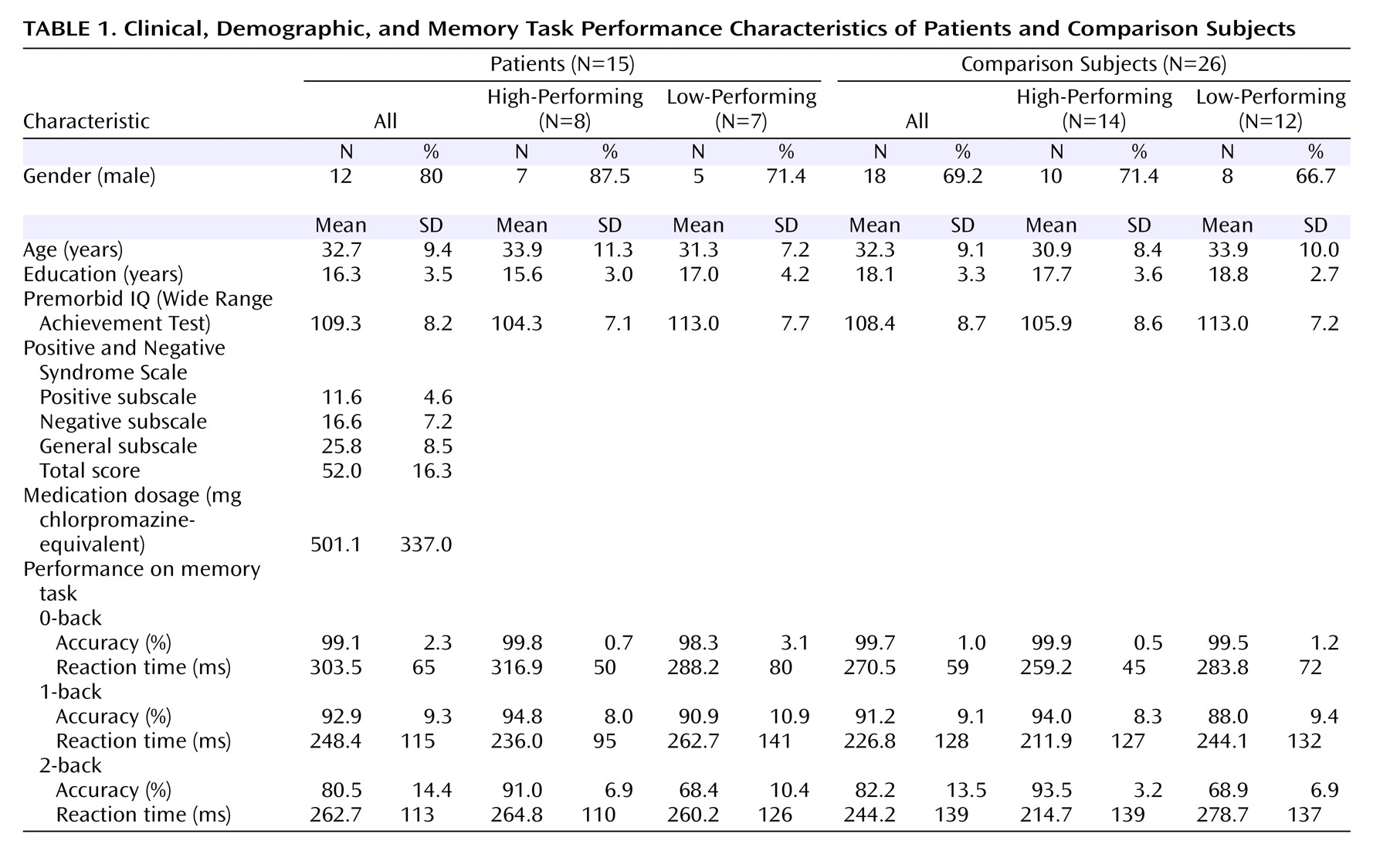
Table 1 ).
There were no significant group differences in age, gender, years of education, or premorbid IQ between the four groups. For N-back reaction time between groups, there were no significant effects of group, working memory load, or group-by-load interaction. As expected, high-performing patients had better accuracy than low-performing patients at 2-back (t=5.0, df=13, p<0.001) but not at 1-back (t=0.79, df=13, n.s.). Similarly, high-performing comparison subjects had better accuracy than low-performing comparison subjects at 2-back (t=11.9, df=24, p<0.001) but not at 1-back (t=1.73, df=24, n.s.).
Within the high-performing cohort, participants maintained high performance accuracy between 1-back and 2-back, with no within-group differences. There were also no accuracy differences between high-performing comparison subjects and high-performing patients at 1-back or 2-back. Within the lower-performing cohort, accuracy was lower at 2-back than at 1-back in each group (low-performing patients: t=4.93, df=6, p=0.003; low-performing comparison subjects: t=7.77, df=11, p<0.001). There were no significant differences in accuracy between low-performing comparison subjects and low-performing patients at 1-back or 2-back.
Single-subject contrast images were entered into a second level of analysis with subject as a random factor. In a preliminary step, we explored the main effect of each working memory task condition (i.e., 1-back or 2-back versus 0-back) for each of the four subgroups and across groups in each performance cohort. We also explored the effects of working memory executive load (2-back versus 1-back) in the combined sample, in all patients versus all comparison subjects, and in pairwise contrasts across the four subgroups. Coordinates were transformed to standard space
(29) and reported as z scores with a significance threshold of p<0.005 (uncorrected), except for the working memory executive load contrast with the combined sample, which was threshold corrected for false-discovery rate
(30) at p<0.05.
In a confirmatory analysis, functional regions of interest in the dorsal and ventral prefrontal cortex were subsequently identified from the working memory executive load contrast involving the combined sample. Note that this contrast was orthogonal and unbiased to the group-by-load interaction effects of interest. Mean fMRI signal intensities were extracted from the individual subject contrast maps at 2-back and 1-back by use of a 10-mm diameter sphere centered on one peak in the dorsal prefrontal cortex and another in the ventral prefrontal cortex. Repeated-measures ANOVA was then used to study these two regions of interest to confirm the main group-by-load interactions identified in the exploratory voxelwise contrasts.
In the functional connectivity analyses, the same dorsal and ventral prefrontal cortex regions of interest were used as seed volumes. From each of these, the median time series was computed for each individual at 2-back and cross-correlated with the time series of all other brain voxels with Pearson’s correlation coefficients. Group differences in functional connectivity were then computed by combining the correlation maps with group as a random factor
(31) . In accordance with our a priori hypothesis and on the basis of known distributed functional and structural anatomy of working memory
(8,
26,
27), our analyses were restricted to the posterior parietal cortex (Brodmann’s areas 7 and 40) by masking through use of WFU PickAtlas software
(32) . The results combining all patient versus all comparison groups are reported and thresholded at p<0.05 with small-volume correction.
Results
Exploratory Voxelwise Contrasts
In each group of patients and comparison subjects, a similar frontoparietal working memory network was activated at 1-back versus 0-back and 2-back versus 0-back. At each load level in the high-performing cohort, patients had greater prefrontal cortex activation than comparison subjects, whereas in the low-performing cohort, patients had regions of lower and greater prefrontal cortex activation compared with comparison subjects, similar to findings reported previously
(17) . A figure containing surface renderings of 2-back activations across performance-matched cohorts is included in a data supplement that accompanies the online version of this article.
The effects of working memory executive load and putatively higher-order working memory complexity were subsequently explored using the contrast 2-back versus 1-back. While the combined groups engaged dorsal and ventral prefrontal cortex to process working memory executive load, patients had relatively greater activation in the left ventral prefrontal cortex. A table listing the regions activated in the combined sample and in each patient and comparison group as well as their between-group contrasts is included in the data supplement that accompanies the online version of this article.
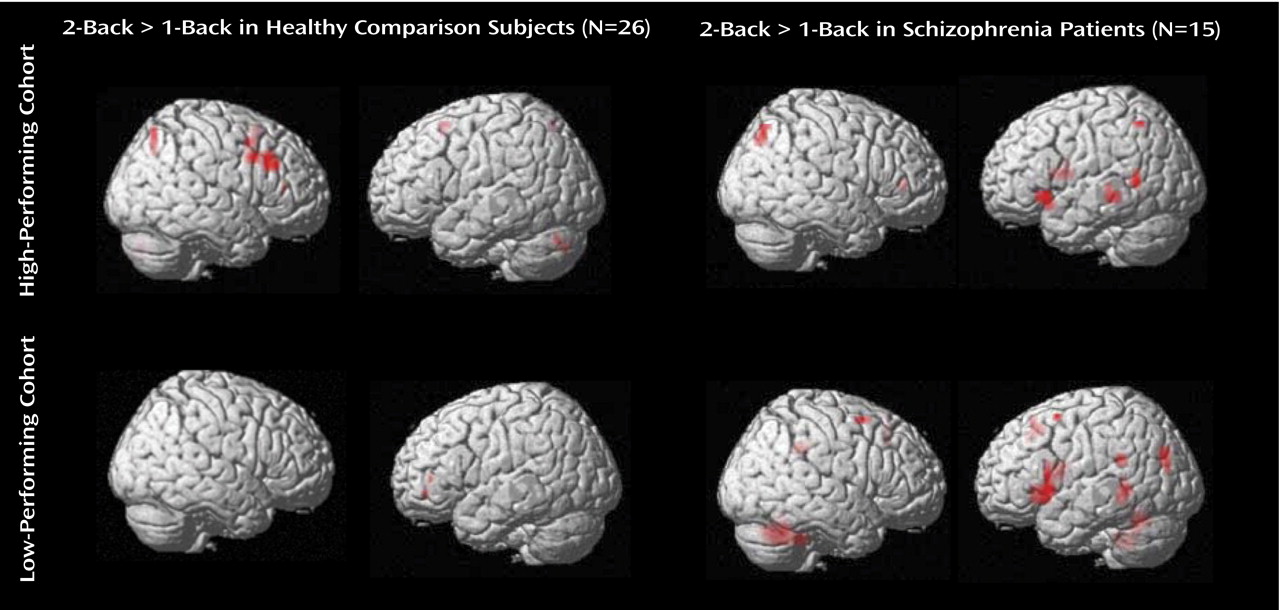
Regions activated in the subgroups and their contrasts are shown in
Figure 1 ; additional details are presented in a table in the data supplement that accompanies the online version of this article. In high-performing comparison subjects, incremental working memory executive load elicited activation in the right dorsal prefrontal cortex. In low-performing comparison subjects, no significant differences in dorsal prefrontal cortex activation were elicited at the chosen threshold. In high-performing patients, an increase in working memory executive load elicited prefrontal activation in the left ventral prefrontal cortex. Similar regions were activated in low-performing patients.
The effects of performance category on working memory executive load were explored in comparison subjects with the contrast of high-performing comparison subjects versus low-performing comparison subjects, and vice versa. The high-performing comparison subjects had a greater increase in activation at the right dorsal prefrontal cortex (peak: Brodmann’s area 46; x=44, y=23, z=27; z score=2.81) relative to low-performing comparison subjects. The ventral prefrontal cortex and reverse contrasts did not elicit significant activations at the chosen threshold.
The effects of performance category on the processing of working memory executive load were explored in schizophrenia patients with the contrast of high-performing patients versus low-performing patients and vice versa. There was no significant increase in activation in the first contrast at the chosen threshold. In the reverse contrast, a region in the left ventral prefrontal cortex had greater activation in low-performing patients compared with high-performing patients (peak: Brodmann’s area 44; x=–55, y=15, z=10; z score=2.75).
The effects of illness on incremental working memory executive load in high-performing subjects were explored with the contrast of high-performing patients versus high-performing comparison subjects and vice versa. A region in the left ventral prefrontal cortex (peak: Brodmann’s area 47; x=–45, y=37, z=–7; z score=2.62) was found to have greater activation in high-performing patients. The reverse contrast did not yield significant activations at the chosen threshold.
The effects of illness on working memory executive load in low-performing subjects were explored with the contrast of low-performing patients versus low-performing comparison subjects and vice versa. A similar region in the left ventral prefrontal cortex (peak: Brodmann’s area 47; x=–30, y=14, z=–2; z score=2.62) was found to have greater activation in low-performing patients. The reverse contrast did not yield activations at the chosen threshold. Note that while the effects reported have so far been leniently thresholded, this result independently replicated that obtained with the high-performing cohort.
Functional Regions-of-Interest Analyses
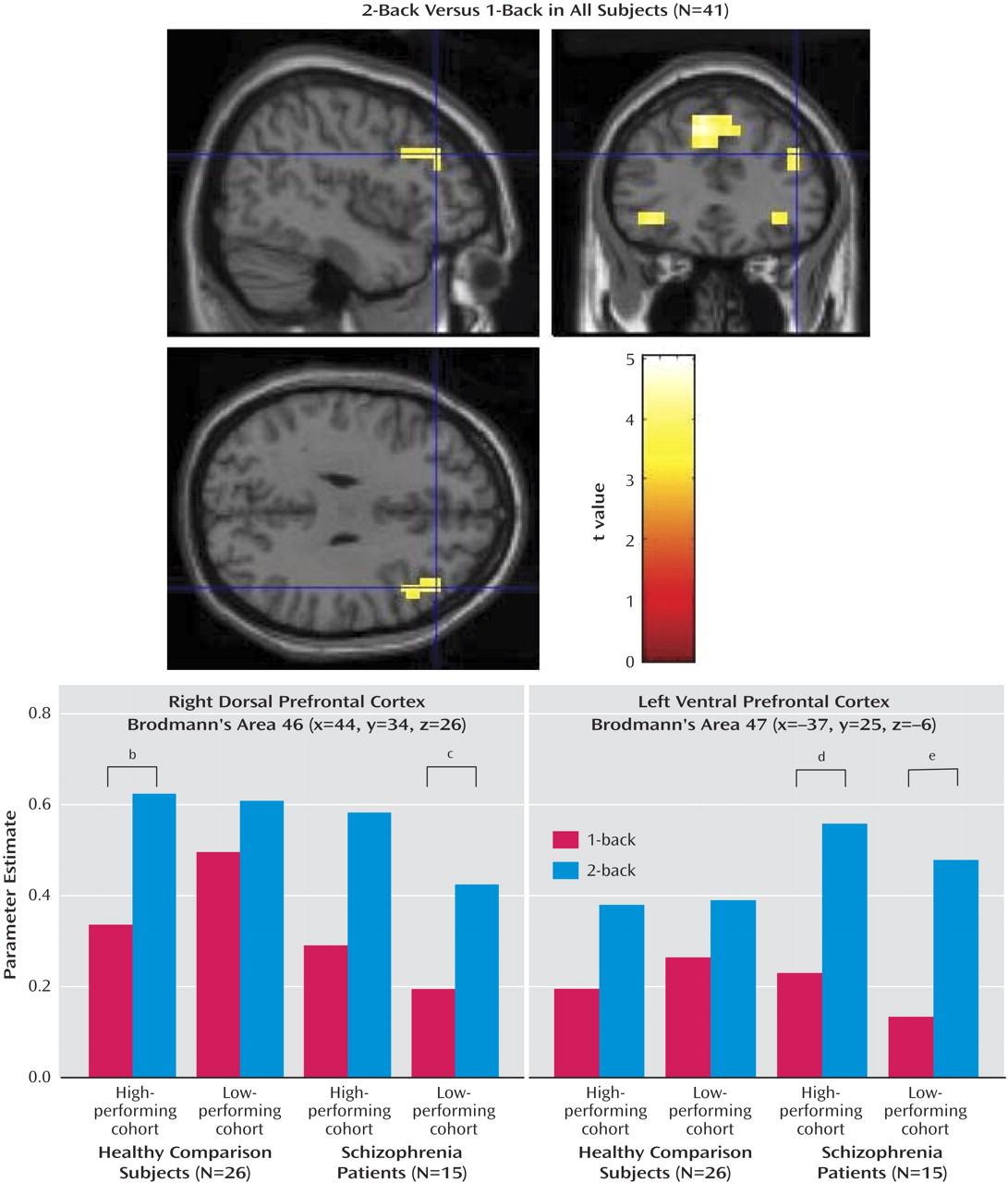
In a confirmatory analysis to reexamine the group effects from the series of exploratory voxelwise contrasts, parameter estimates for each subject were extracted from ventral and dorsal prefrontal cortex regions identified in an orthogonal contrast of 2-back versus 1-back combining all subjects (see
Figure 2 ; see also the first table in the data supplement that accompanies the online version of this article). The parameter estimates for each N-back task condition in the right dorsal prefrontal cortex (Brodmann’s area 46; x=44, y=34, z=26) showed an effect of load in all subjects (F=33.3, df=1, 37, p<0.001), and, in comparison subjects, effects of load (F=31.4, df=1, 24, p<0.001) and load-by-performance-group interaction (F=6.0, df=1, 24, p=0.022). Here, consistent with the voxelwise contrasts, high-performing comparison subjects had a disproportionately greater increase in activation between 1-back and 2-back relative to low-performing comparison subjects (see
Figure 2, lower left panel). Parameter estimates from the left ventral prefrontal cortex (Brodmann’s area 47; x=–37, y=25, z=–6) yielded load effects in all subjects (F=55.2, df=1, 37, p<0.001), driven by a load-by-group interaction in which patients had disproportionately increased activation (F=7.51, df=1, 37, p=0.009) (see
Figure 2, lower right panel). This result was also consistent with the voxelwise contrasts. In addition, there was a group-by-region interaction (F=7.61, df=1, 39, p=0.009) in which comparison subjects had greater regional activation at the dorsal relative to the ventral prefrontal cortex, whereas patients lacked this regional differentiation and had greater ventral prefrontal cortex activation than comparison subjects. Finally, in high-performing comparison subjects, 2-back activation at the dorsal prefrontal cortex region of interest correlated with performance accuracy (r=0.48, p=0.041), but not at the ventral prefrontal cortex region of interest (r=–0.23, n.s.). In patients in both performance cohorts, ventral prefrontal cortex activation correlated with accuracy (r=0.52, p=0.024), but not dorsal prefrontal cortex activation (r=–0.19, n.s.).
Functional Connectivity Analyses
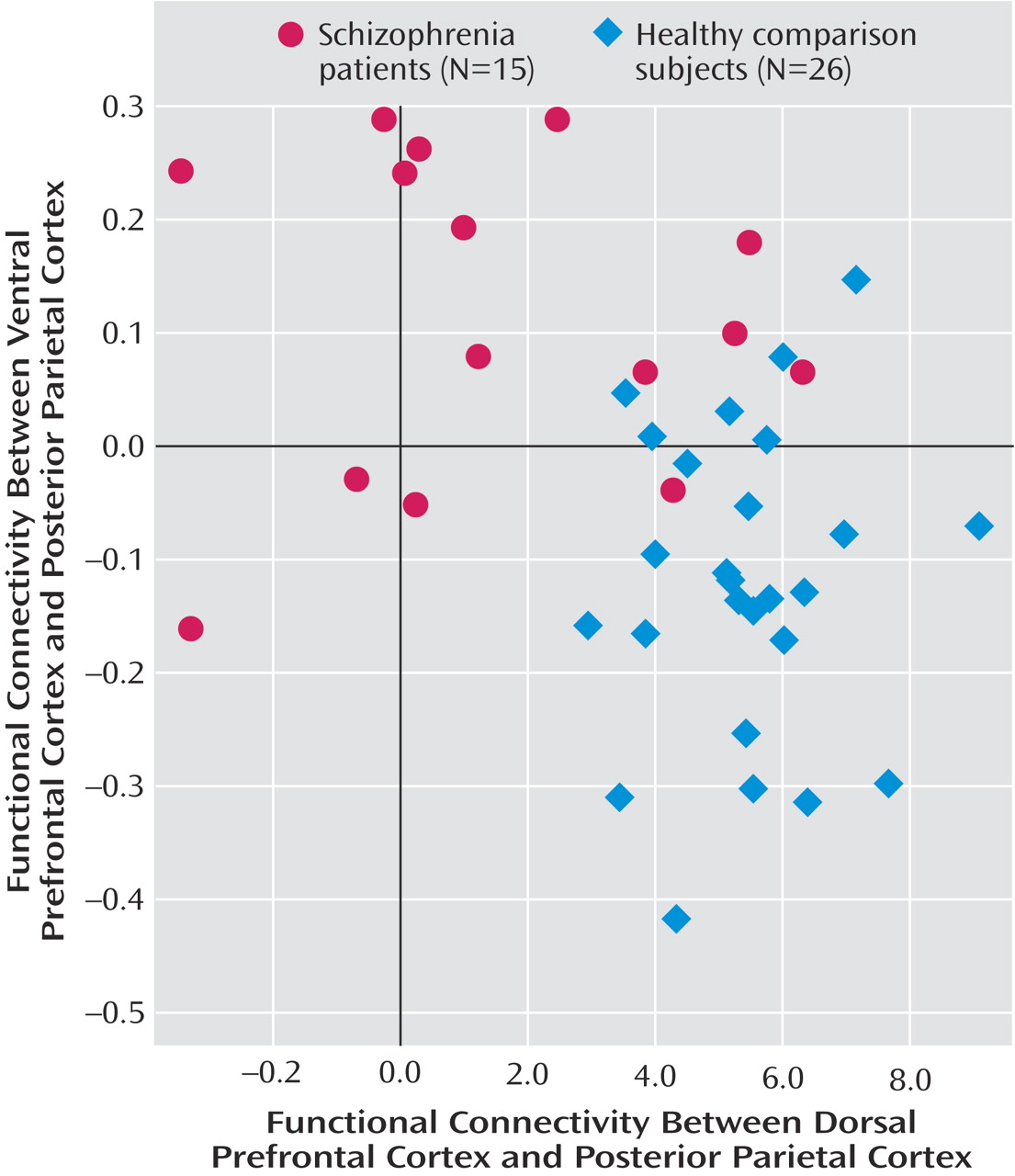
In the functional connectivity analyses, seed regions were derived from regions of interest in the dorsal and ventral prefrontal cortex. In comparison subjects relative to patients, the dorsal prefrontal cortex region of interest had greater functional connectivity with the bilateral inferior parietal lobules (Brodmann’s area 40; x=34, y=–64, z=54; z score=4.87; and x=–49, y=–49, z=48; z score=4.39). Conversely, in patients relative to comparison subjects, the ventral prefrontal cortex region of interest had greater connectivity with the left superior parietal lobule (Brodmann’s area 7; x=–11, y=–82, z=42; z score=3.90). For each individual subject, the mean correlation coefficients for the connectivity between the respective right dorsal prefrontal cortex and posterior parietal cortex and the left ventral prefrontal cortex and posterior parietal cortex were subsequently extracted from 10-mm diameter spheres centered on the peaks at the respective right (x= –34, y=–64, z=54) and left posterior parietal cortex (x=–11, y=–82, z=42). The functional connectivity levels of these regions were inversely correlated with each other, with lower connectivity between the dorsal prefrontal cortex and posterior parietal cortex predicting higher connectivity between the ventral prefrontal cortex and posterior parietal cortex, and vice versa (r=–0.41, p=0.008) (
Figure 3 ). Correlations within each group, however, did not reach statistical significance.