In postmortem studies of patients with autism, abnormalities are found in the limbic cortex, the cerebellum, and the white matter of the brain, but not in the frontal cortex, the basal ganglia, or the thalamus
(1) . Subtle changes in frontal cortical organization
(2,
3) and greater cerebral gray matter volume
(4) have also been observed. By contrast, clinical observations in patients with autism reveal stereotypies
(5 –
7), gross motor function abnormalities
(8), reward system deficits, and impairment in auditory, tactile, and visual sensory stimuli processing
(9,
10), which are suggestive of functional impairments in the caudate, the putamen, and the thalamus. Disordered reward systems have been reported to have an influence on stereotypies
(11) and sensory stimulation
(12) in autism, which is also consistent with a possible striatal deficit. Lastly, the serotonin- and dopamine-rich striatum is affected by atypical antipsychotics and serotonin reuptake inhibitors
(13), which are frequently used in the treatment of autism.
Among magnetic resonance imaging (MRI) studies comparing patients with autism and comparison subjects, one study reported a larger caudate
(14) with no change in putamen size in the autistic group, whereas other studies found no difference between patients and comparison subjects in the size of the striatum
(15 –
19) . One computerized tomography study reported smaller caudate volumes in autistic patients relative to comparison subjects
(20), and a study of a twin pair discordant for autism found a smaller caudate in the affected twin
(21) . Studies of the thalamus in autism were also divided, with one study finding lower volumes in autistic patients
(22) and two studies finding no significant differences between patients and comparison subjects
(18,
20) .
We reported previously
(28,
29) that we found no volumetric or metabolic differences between patients with autism spectrum disorders and comparison subjects in the hippocampus and amygdala. However, in the same cohort, the autism spectrum patients had smaller right anterior cingulate volumes and lower glucose metabolic rates bilaterally in the anterior cingulate during performance of a verbal memory task. The cortico-striato-thalamic loop has been posited as critical in sensory regulation
(30), and the pattern of abnormalities we observed suggested the need to examine the next way stations in the circuit. Pathways from the anterior cingulate pass via the ventral striatum to the thalamus
(31), and the anterior cingulate appears to be closely connected to the anterior thalamus
(32 –
34) . In the neural circuits proposed by Alexander et al.
(31,
35,
36) —loops involving frontal lobe, cingulate, striatum, and thalamus—a distinction is made between the dorsal and ventral striatum. This distinction appears to be of potential interest in autism.
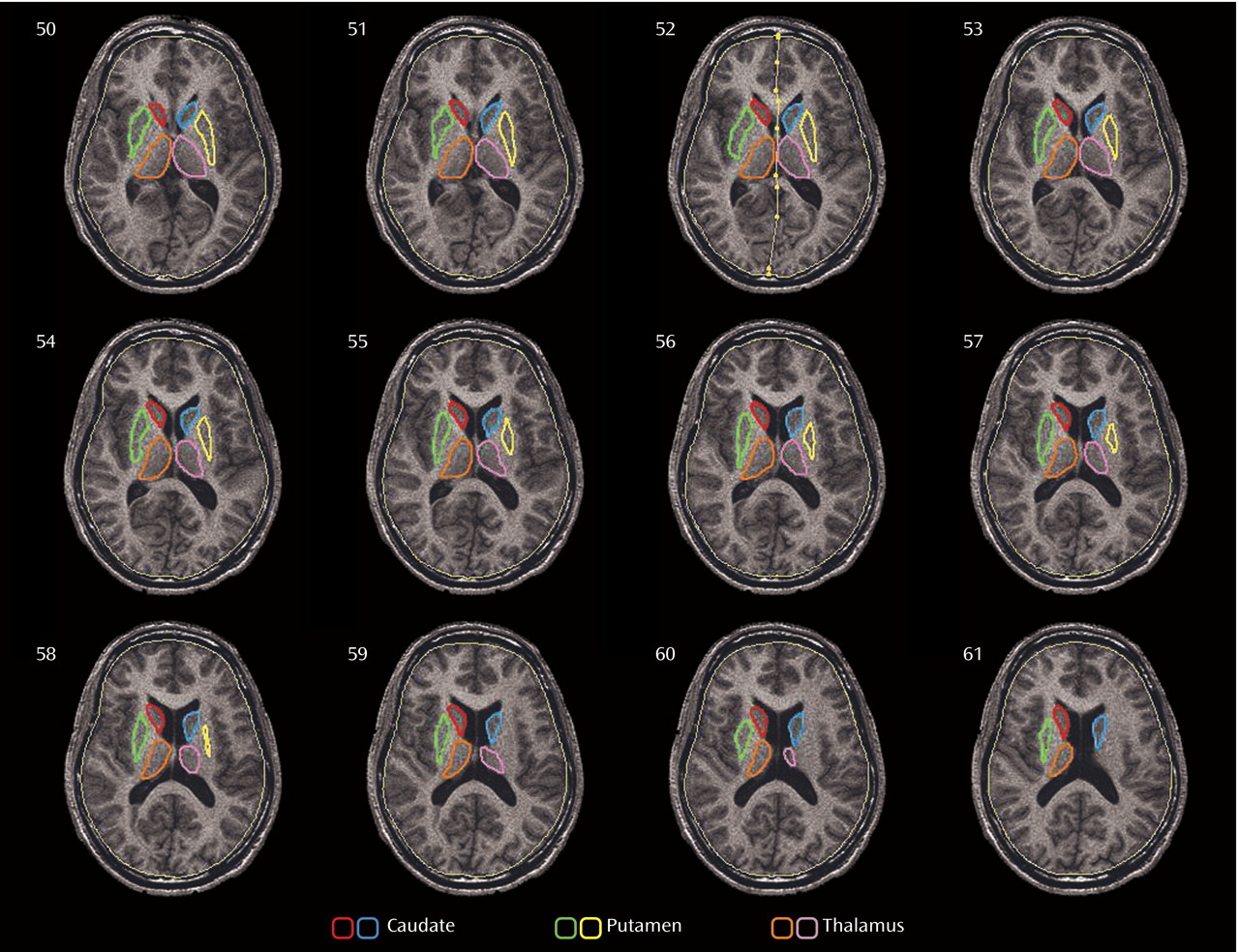
Our aim in this study was to further investigate the dorsal-ventral anatomic distinction, which has not been a focus of previous studies of the striatum in autism. Using data from the same cohort we reported on earlier
(29), we outlined structures on contiguous axial slices of high-resolution MR images and reconstructed regions of interest to yield three-dimensional maps of volumetric and metabolic differences within the entire dorsoventral extent of these structures. We hypothesized that caudate volumes would be larger in patients with autism spectrum disorders than in comparison subjects, as we reported previously
(29) . Because functional images were acquired during the performance of a verbal learning task, we also expected to observe differences between patients and comparison subjects in relative glucose metabolic rate in the anterior cingulate-striatum-thalamus circuit.
Discussion
Findings of larger brain volumes in autistic patients compared with healthy subjects have been reported in some (e.g., reference
49 ) but not all (e.g., reference
50 ) studies investigating this issue. Recent studies have found that total brain volume is higher in autistic patients when they are young children but that among adults, brain volumes are indistinguishable between patients and comparison subjects
(51 –
54) . In our cohort of adult patients, no group differences were observed in total brain volume
(29), which allowed us to examine the volumes of structures both in absolute terms (measured in mm
3 ) and relative to total brain volume to remove individual differences in body size.
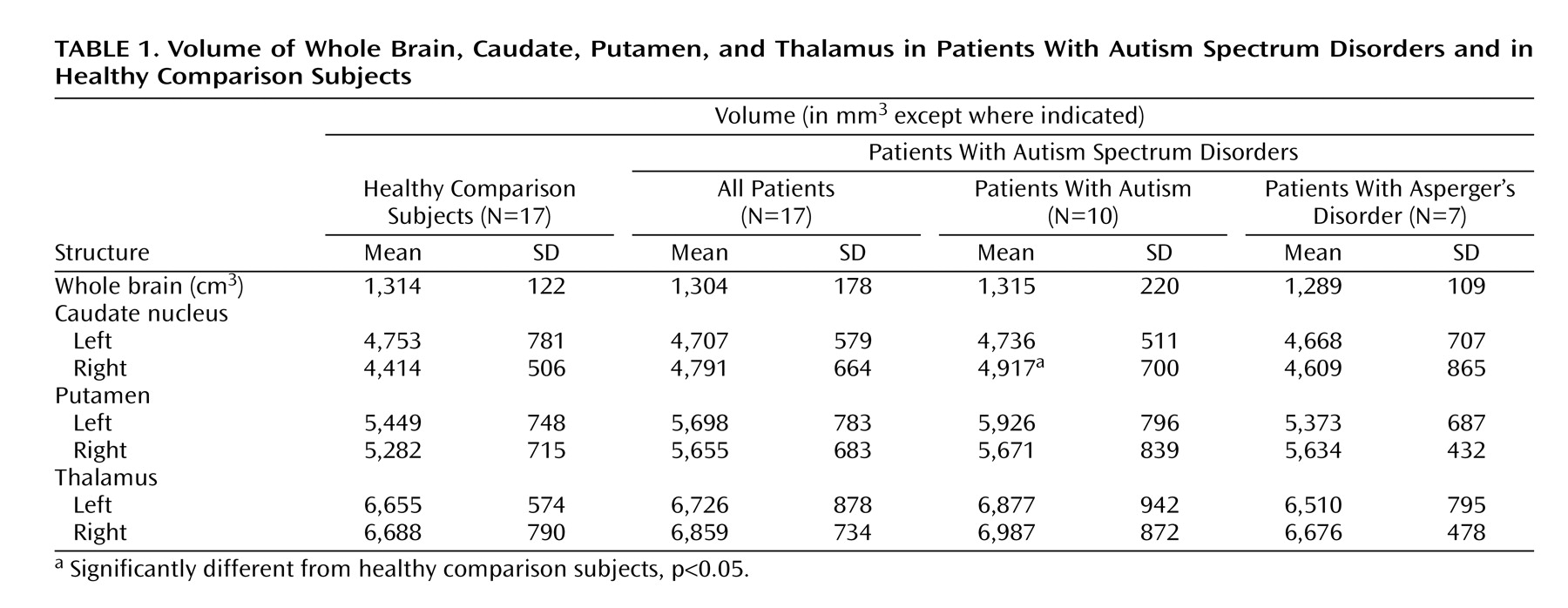
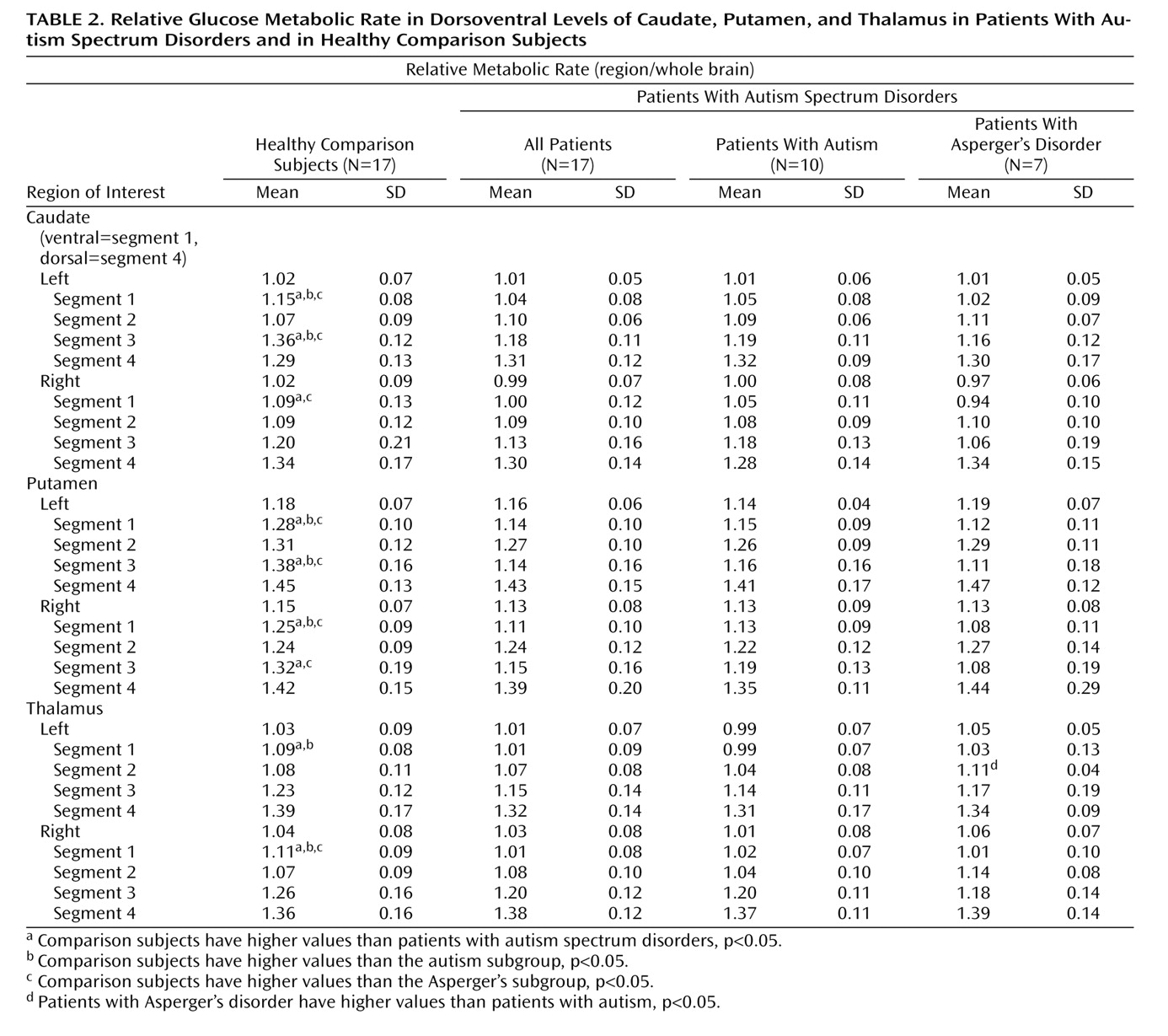
The absolute volume of the right caudate was significantly greater in the autistic subgroup than in the comparison subjects, but the greater volume was not significant for the entire autism spectrum disorders group. The effect size for the right caudate for all 17 patients was 0.64, similar to the 0.61 effect size we calculated from data presented in an earlier report showing significantly larger caudate volumes among patients
(14) . However, with our group size of 17, we had a power of only 42% to detect this size effect, whereas the other study, with N=35, had a power of 74%.
Using data from a study in which nonsignificant caudate enlargement was reported
(16), we again calculated a caudate effect size of 0.61, but the power in that study was only 41%. In a third study
(17), in which nonsignificant enlargement was reported in the right caudate in patients with Asperger’s disorder, the effect size was only 0.14 (comparable to our effect size of 0.28 for this subgroup), although correction for intracranial volume reduced the effect size to 0.0 in their data. A fourth study
(18) had a very small negative effect size for the right caudate (–0.02). In our data, the finding of right caudate enlargement in the autism spectrum disorders group was not affected by partialing brain volume, and it gained significance when body surface area was used as a covariate. As others have reported in MRI studies
(14,
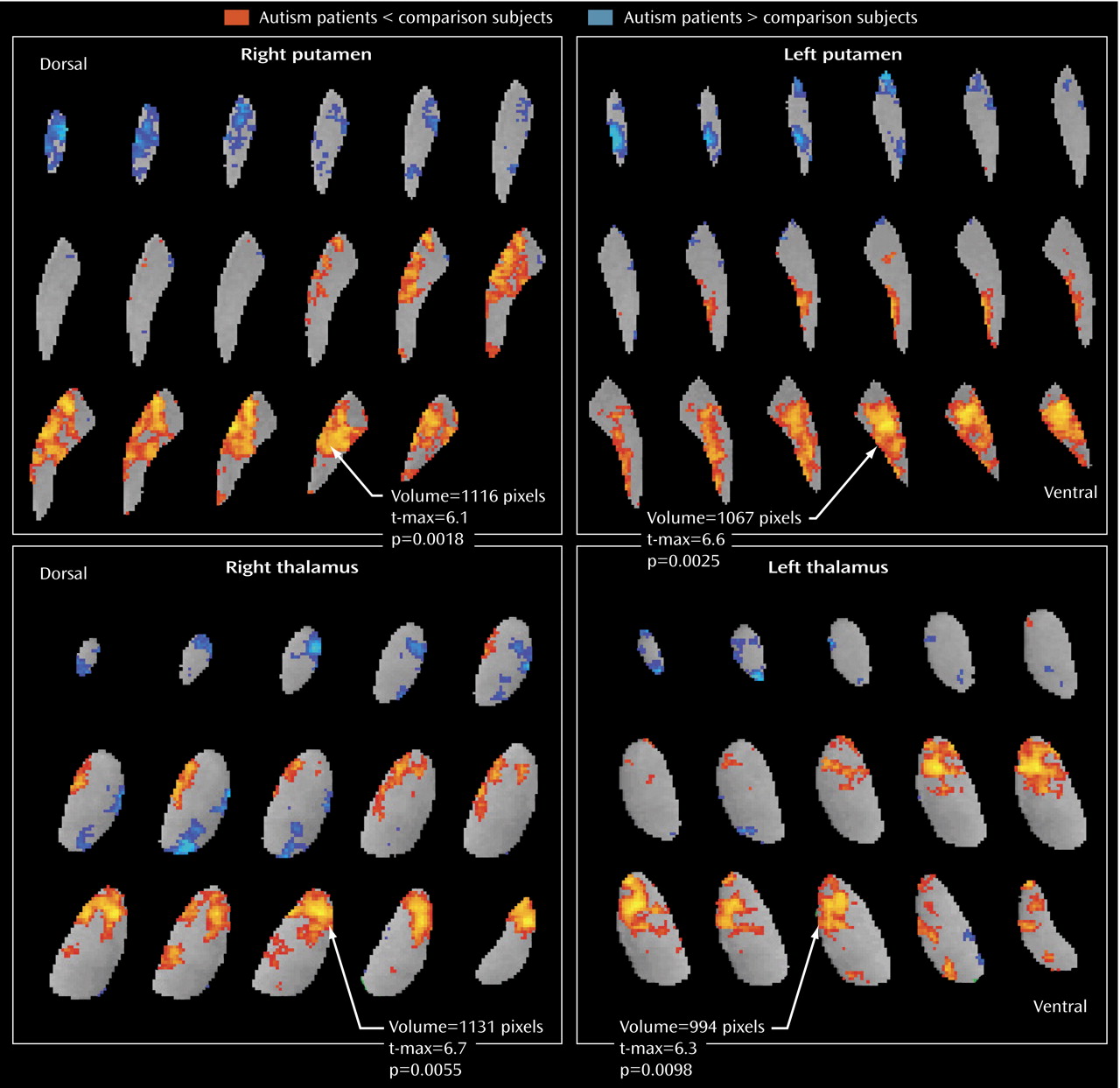
18), we found no group differences in volumes of the putamen or thalamus.
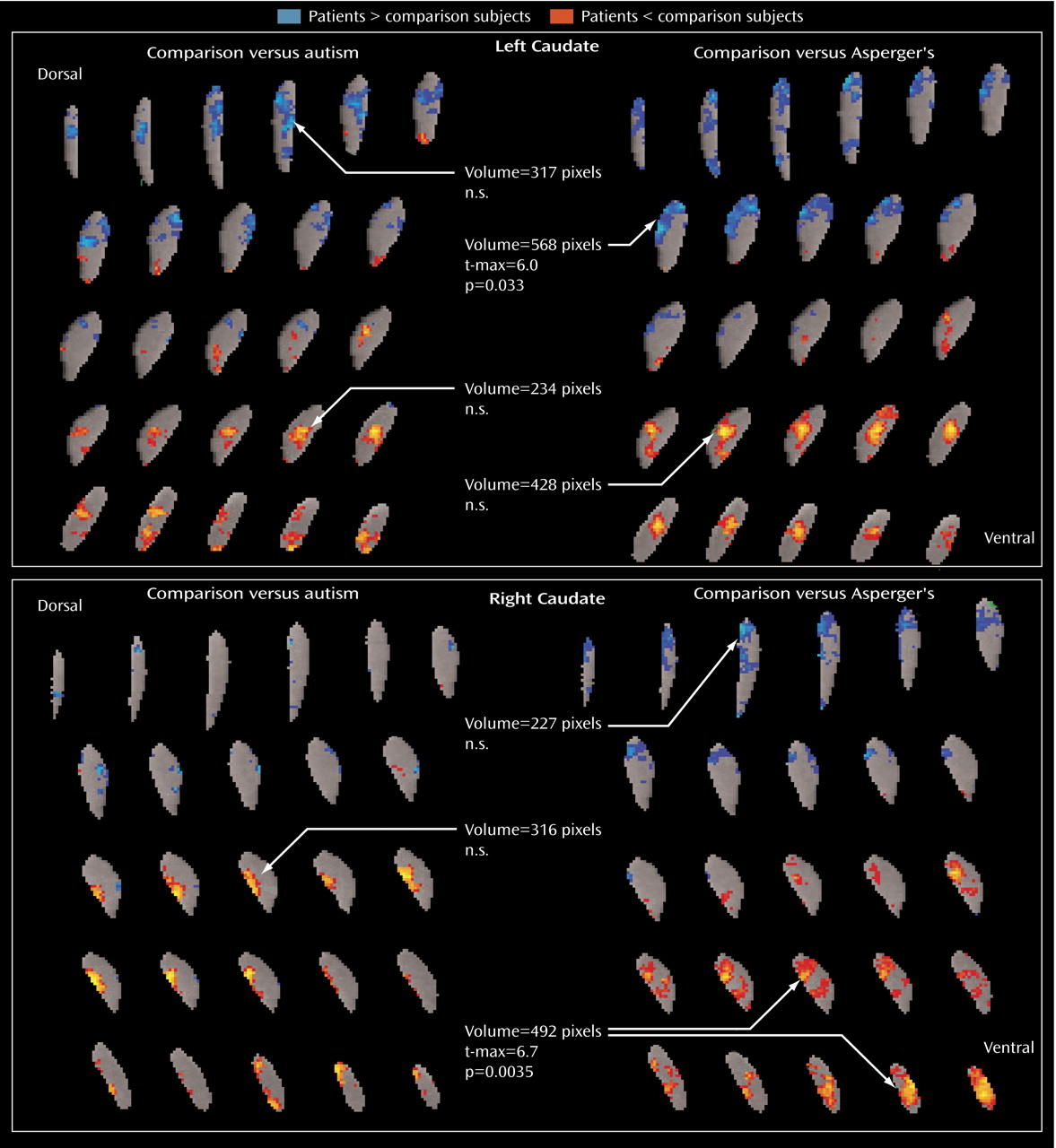
The finding of larger caudate volumes cannot be explained by exposure to antipsychotic medication; only two of the autism spectrum disorder patients had a history of exposure to antipsychotics, and neither had used antipsychotics within the previous year. Previously, our group reported correlations between higher-order repetitive behavior and right caudate enlargement in a group of autism spectrum patients, most of whom are included in this study. Caudate enlargement has also been reported in patients with somatization disorder
(56) and in patients with schizophrenia who have been treated with antipsychotics
(45) . In the absence of treatment with antipsychotics and differences in total brain volume, the enlargement of the right caudate in the autistic subgroup, coupled with an altered metabolic pattern in the caudate and thalamus, suggests a disturbance in striatothalamic circuitry. This finding requires replication, however, because of the small sample size (N=10).
These striatothalamic alterations, along with the failure of the ventral striatum to activate while subjects are performing a task involving verbal memory and semantic categorization, point to a deficit in frontal-cingulate-striatal-thalamic interconnections
(57) . A task that involved specific rule-based semantic categorization in normal subjects
(57) activated the caudate ventrally (z=–4), while a similarity-based categorization (a comparison with previously encountered examples) evoked caudate activation at a slightly higher level (z=0). Both of these points are relatively ventral, since our tracing extends 2 cm higher (z=20–24). These findings suggest the importance of the caudate for resource-demanding tasks, helping support working-memory demands. This interpretation of the role of the caudate in semantic memory is consistent with the heavy memory load imposed by the verbal memory task in this study.
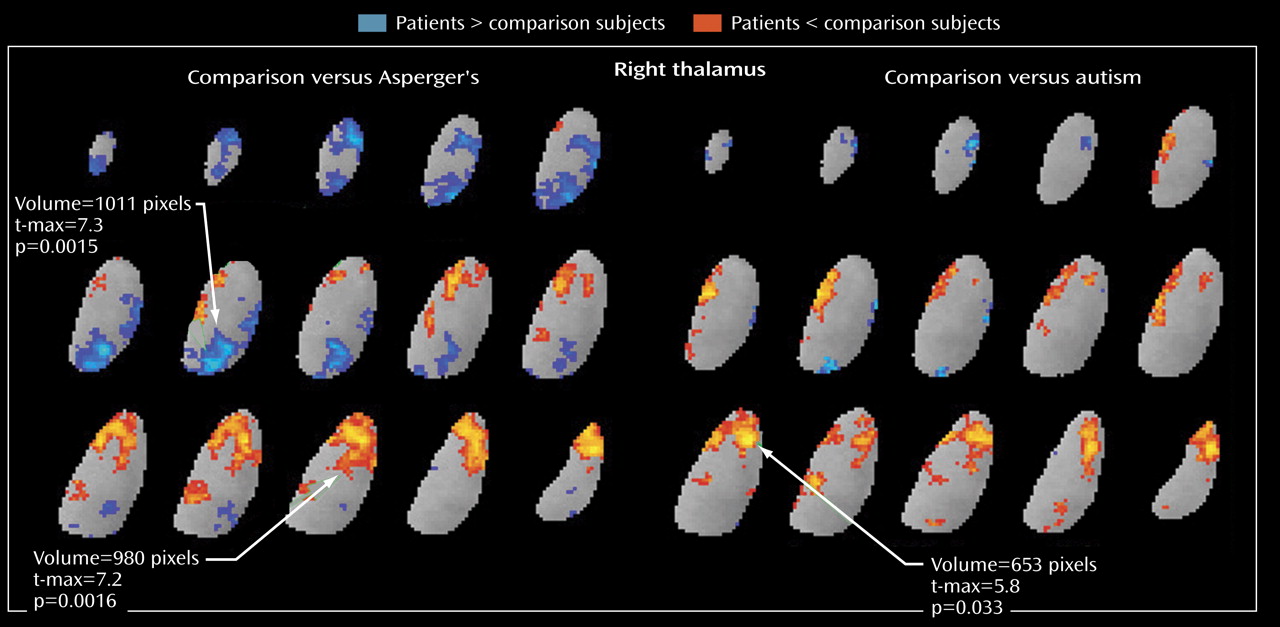
The area of metabolic hypoactivity that we observed in the thalamus appears to be in the anterior nucleus, which is closely connected to the anterior cingulate cortex
(32 –
34) . We reported lower glucose metabolism in the anterior cingulate for the same cohort
(28,
29), and another group reported that bipolar patients who performed poorly on the California Verbal Learning Test, which is closely analogous to the task used here, were found to have a lower gray matter density in the anterior cingulate
(58) . In the present study, two findings combine to suggest an impairment in the function of this circuit in autistic patients. First, in our comparison group, the ability to semantically categorize the word list was correlated with the relative metabolic rates of both the thalamus and the putamen, whereas the patients with autism spectrum disorders showed no such correlations, which is consistent with disturbed coupling. Interestingly, unilateral lesions of the anterior thalamus in monkeys impair assessment of semantic knowledge
(59) . The left anterior thalamus has also been reported to show greater activation during encoding of complex semantic distinctions in human fMRI studies
(60) . Second, the finding of low metabolic activity in the anterior cingulate, ventral (but not dorsal) striatum, and anterior thalamus but not the pulvinar, temporal cortex, hippocampus, or amygdala, and in medial but not dorsolateral cortex
(61), links these structures. Our findings are not inconsistent with previous reports on the functional alteration of the dentato-thalamo-cortical pathway in autism
(62) .
Activation of the pulvinar may be a compensatory mechanism to moderate deficits in patients with Asperger’s disorder. While both the patients with autism and those with Asperger’s disorder had low relative metabolic rates in the anterior thalamic nucleus, rates in the medial dorsal nuclei and the pulvinar were even higher in the Asperger’s subgroup than in the comparison group. Asperger’s patients did not differ from comparison subjects in their ability to semantically categorize the word list, possibly because they are able to use other circuitries successfully (e.g., the mediodorsal thalamic nuclei-frontal cortex or the pulvinar-temporal lobe). This pulvinar-mediodorsal activity (the blue areas in
Figure 5 ) may reflect an important compensatory response involved in ameliorating anterior thalamic deficits.
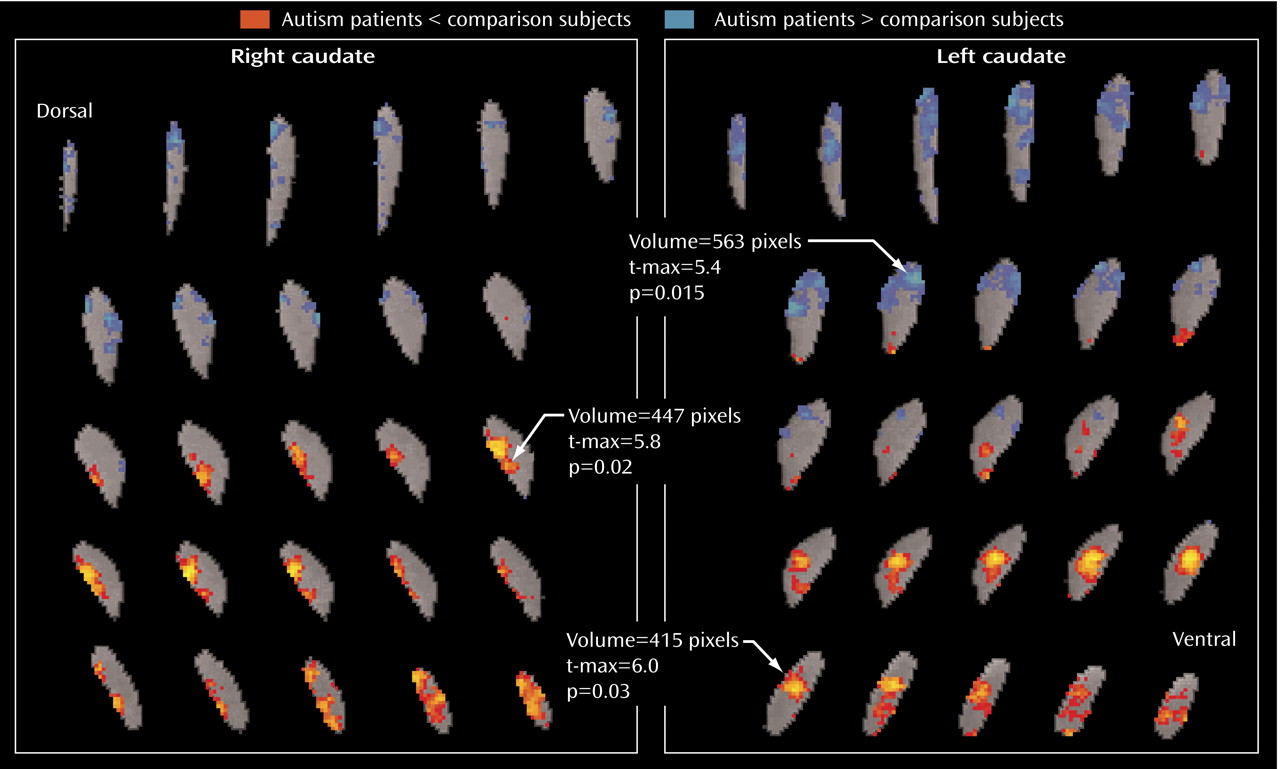
The metabolic activity of the dorsal and ventral caudate nucleus differed markedly between our autism spectrum disorder patients and our comparison subjects. The patient group had lower relative glucose metabolic rates ventrally but higher rates dorsally, which is consistent with the differential roles of the ventral and dorsal striatum, as reviewed above. Dorsal activation, by contrast, might have been expected in a sensory task, such as the continuous performance task or a reaction-time task. Alternatively, if reward aspects are the most important, autistic patients might exhibit both ventral and dorsal deficits when continuous performance or reaction-time tasks are performed but only ventral deficits in tasks that engage ventral cognitive circuits. Also interesting to note is that the Asperger’s patients had higher metabolic activity than comparison subjects in the left dorsal caudate, which is similar to the metabolic pattern seen in obsessive-compulsive disorder, especially in patients who respond to serotonin reuptake inhibitors
(63,
64) .
Limitations of this study include its relatively small sample size, the overrepresentation of verbally competent patients, the lack of Autism Diagnostic Observation Schedule data on our patient group, and the resolution limits of [
18 F]fluorodeoxyglucose. While this study had adequate power to detect large effects (85% power to detect an effect size of 1.0), its power to detect subgroup differences was much lower. The patients who participated in this study are representative of approximately 25% of the autism spectrum, and they all have verbal communication skills, relatively high cognitive functions, and no severe neurological impairments. Thus, the findings of this study cannot be generalized to the entire autism spectrum. Also, our division of patients into the autism and Asperger’s subgroups is limited by the lack of structured criteria for this distinction in adults, and our groupings may merely reflect illness severity. The differences between these groups in cognitive abilities were statistically significant, but we did not match patients and comparison subjects on IQ. Although previous studies have not reported lower metabolic activity in the basal ganglia in patients with mental retardation
(65), our findings could have been confounded by the group differences in IQ. Cognitive abilities in autism and Asperger’s disorder also may show complex patterns of individual deficits, and much larger groups would be necessary to separate global deficits from specific cognitive strengths and weaknesses. Lastly, our PET resolution full width at half maximum of 4.5 mm in-plane and 6.5 mm axially is not large relative to portions of the caudate and thalamus. The caudate, 30 mm high and 10 mm at its widest extent ventrally (z=1)
(66), is larger than the amygdala. The anterior thalamic areas probably also include anterior parts of the medial dorsal and ventrolateral nuclei. Enlargement of the third ventricle, as reported elsewhere
(67), might also contribute to diminished anterior activity through partial volume effects, but given that the edge shadow of this structure does not appear in
Figure 3 and
Figure 5, such an explanation is less likely.