Antipsychotic medications have been the first-line treatment for psychotic disorders for nearly half a century
(1) . Despite this long history of use, many patients dislike taking the medications and are eager to stop them
(2) . As early as 1957 it was reported that 25% of normal subjects given only 50 mg of chlorpromazine experienced “inability to think, tiredness, sleepiness and generally unpleasant feelings”
(3) . Over the years a number of reports have commented on this adverse subjective experience of antipsychotics using a diversity of terms: “behavioral toxicity,” “neuroleptic dysphoria,” “akinetic depression,” “subjective extrapyramidal side effects,” and “neuroleptic-induced deficit syndrome” (reviewed by Voruganti and Awad
[4] ). While these adverse subjective effects are well documented and it is recognized that subjective experiences are key factors for adherence and recovery
(2), little is known about their underlying neurobiology.
In recent years, the disruption of the dopamine system has been associated with these subjective experiences
(4) . For example, alpha-methyl paratyrosine (AMPT)—an agent that blocks catecholamine synthesis and decreases dopamine levels temporarily—has been shown to produce reversible dysphoria, decrease in happiness, and tiredness in healthy volunteers
(5,
6) and in patients with schizophrenia
(7) . Further, it has been demonstrated that high levels of dopamine D
2 blockade (as measured using SPECT) is associated with dysphoric experiences
(8,
9) or depression
(10) in patients with psychosis treated with antipsychotic medications. For example, de Haan used the Subjective Well-Being Under Neuroleptics scale, which reliably measures the subjective experience associated with the use of antipsychotics, and found a significant association between subjective experience and striatal D
2 receptor occupancy in schizophrenic patients clinically stable on regimens of atypical antipsychotics. Even in the absence of extrapyramidal symptoms, higher striatal D
2 receptor occupancy by olanzapine and risperidone was related to worse subjective experience, more severe negative symptoms, and depression.
While the aforementioned data implicate a blockade of dopamine transmission, particularly at the dopamine D
2 receptor, as a precursor of adverse subjective effects, all of these studies have examined only the “striatal” dopamine D
2 receptors. Dopamine D
2 receptors are expressed in several brain regions (thalamus, prefrontal cortex, temporal cortex) beyond the striatum
(11), and these “extrastriatal” regions are of particular relevance to subjective experience
(12) . While the striatal regions have been associated with the motor side effects of antipsychotic medications, it is the thalamic, prefrontal, and limbic regions that are more directly implicated in cognition, reward, and motivation
(13 –
16) . However, measuring extrastriatal D
2 receptors poses a technical challenge, since the levels of dopamine D
2 receptors are much lower (a tenth to a hundredth) than in the striatum
(11) . [
11 C]FLB 457 is a high affinity ligand that can reliably measure regions with low receptor density (i.e., extrastriatal regions), but it does not reach equilibrium in the striatal regions (i.e., high density) within the 60–90 minute time window that is feasible with [
11 C] ligands
(17,
18), hence necessitating [
11 C]raclopride scans.
In light of these developments, we present the results of the first systematic, double-blind, prospectively controlled study examining the relationship between striatal and extrastriatal D 2 blockade and the adverse subjective experience induced by antipsychotics.
Results
Twelve patients (11 men and one woman) were included in the present study, of whom eight were neuroleptic naive before randomization. Their mean age was 27 years (range=17–45), and they had a mean duration of psychotic symptoms of 98 weeks (range=4–530). Nine of these patients met the criteria for schizophrenia and three met the criteria for provisional schizophreniform disorder at the time of inclusion in the study. Five subjects received risperidone (either 1 mg [N=2] or 4 mg [N=3]) and seven received olanzapine (either 2.5 mg [N=3] or 15 mg [N=4]). On the day of the PET scan the patients exhibited mild/moderate severity according to the CGI severity ratings (mean score=3.7, SD=0.67), and the mean PANSS score was 65.90 (SD=8.3). The mean PANSS positive and negative symptom scores were 15.10 (SD=2.6) and 15.60 (SD=5.3), respectively. Simpson-Angus Rating Scale score was 3.62 (SD=4.86), and the Barnes Akathisia Scale score was 0.37 (SD=1.09). The Subjective Well-Being Under Neuroleptics scale total score was 87.25 (SD=17.51) and was not associated with PANSS positive symptom scores (r= 0.11, p=0.78). Subjective Well-Being Under Neuroleptics total score was significantly correlated with Simpson-Angus Rating Scale score (r=–0.71, p=0.047) but not with the Barnes Akathisia Scale score (r= –0.34, p=0.37). Two subjects who had the highest Simpson-Angus Rating Scale scores received benztropine.
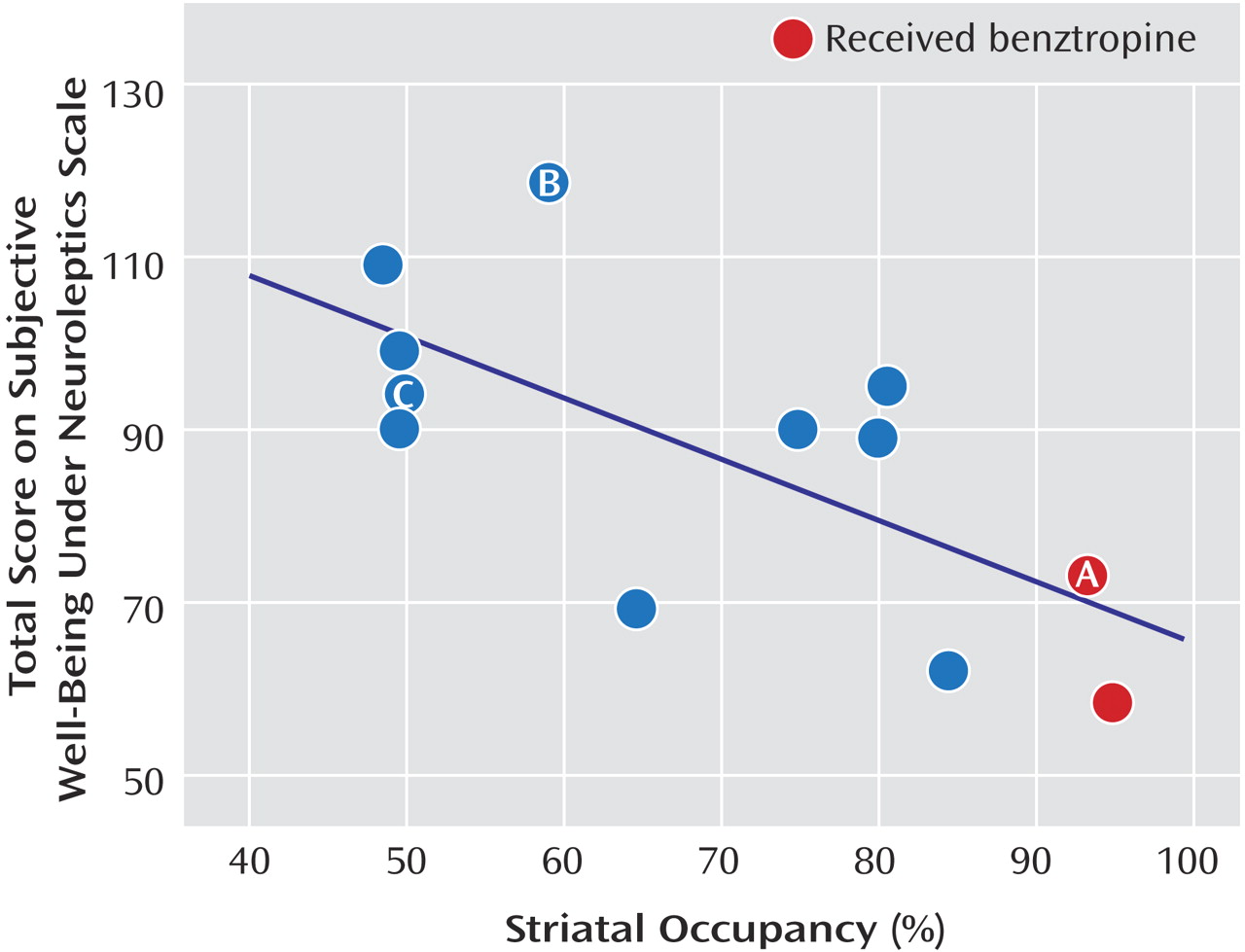
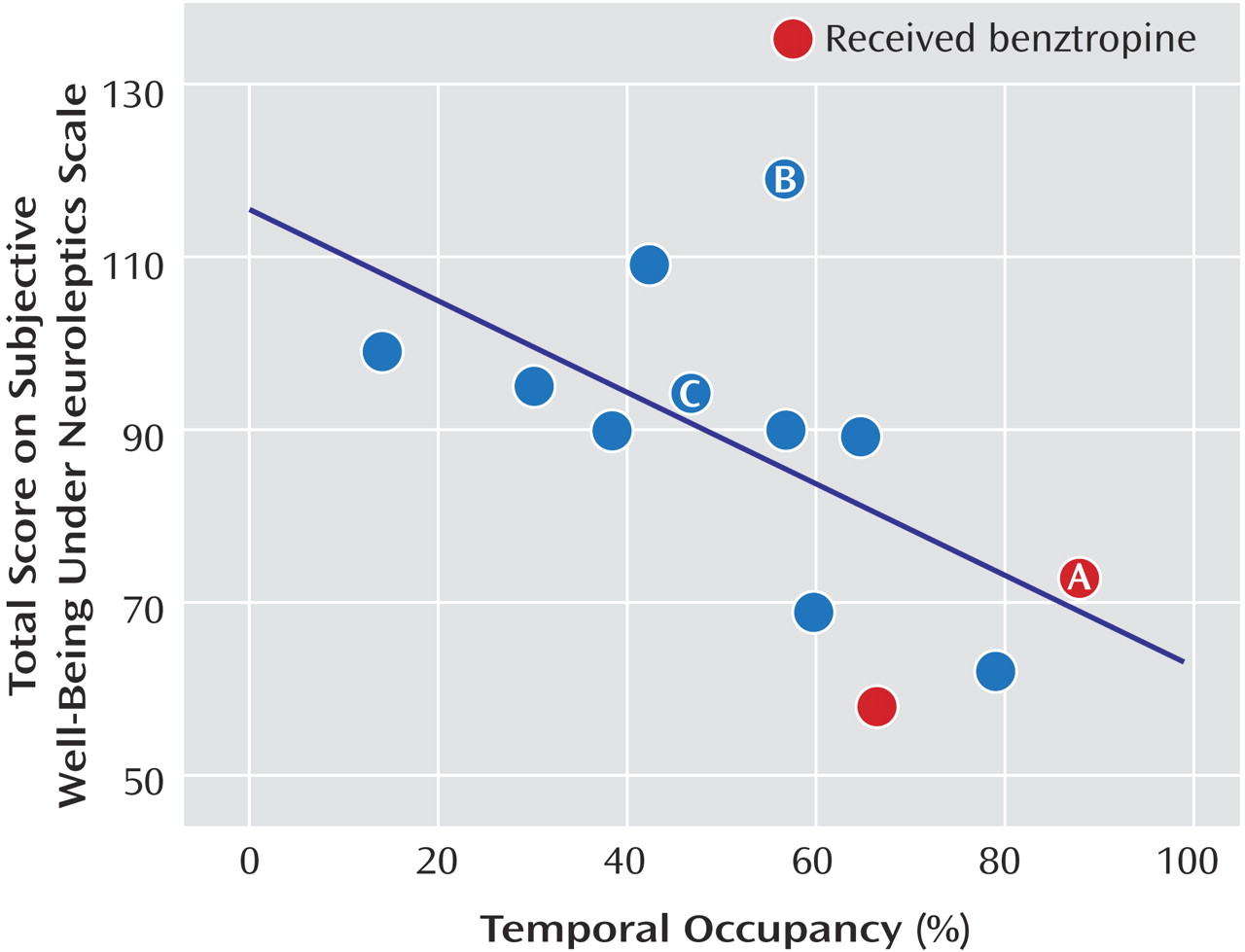
The measured D
2 occupancies ranged from 50%–91% in striatal and 4%–95% in the different extrastriatal regions. The Subjective Well-Being Under Neuroleptics scale total score was significantly associated with striatal occupancy (r=–0.66, p=0.01 [
Figure 1 ]) and temporal occupancy (r=–0.76, p=0.003 [
Figure 2 ]) but not frontal or thalamic occupancy. These associations remained unchanged when subjects were divided by those that were treated with either risperidone or olanzapine, excluded those that received previous antipsychotic treatment, or those with the highest Simpson-Angus Rating Scale scores. To further investigate these associations between striatal and temporal occupancy with subjective experience, we performed stepwise regression analysis with the Subjective Well-Being Under Neuroleptics subscales to avoid multiple comparisons. A significant association (F=12.75, df=1, 10, p=0.005) was seen between striatal blockade and mental functioning (β=-0.74, t=–3.57, p=0.005) but not self-control (β=–0.05, t=–0.20, p=0.84), emotional regulation (β=–0.11, t=–0.36, p=0.72), physical functioning (β=–0.10, t=–0.22, p=0.82), or social integration (β= –0.15, t=–0.50, p=0.62). A significant association (F=8.73, df=1, 10, p=0.01) was seen between temporal blockade and emotional regulation (β=–0.68, t=–2.95, p=0.01), but self-control (β=–0.01, t=–0.03, p=0.97), mental functioning (β=–0.29, t=–0.83, p=0.42), physical functioning (β= –0.14, t=–0.43, p=0.67), and social integration (β=–0.30, t=–1.03, p=0.32) were excluded from the model.
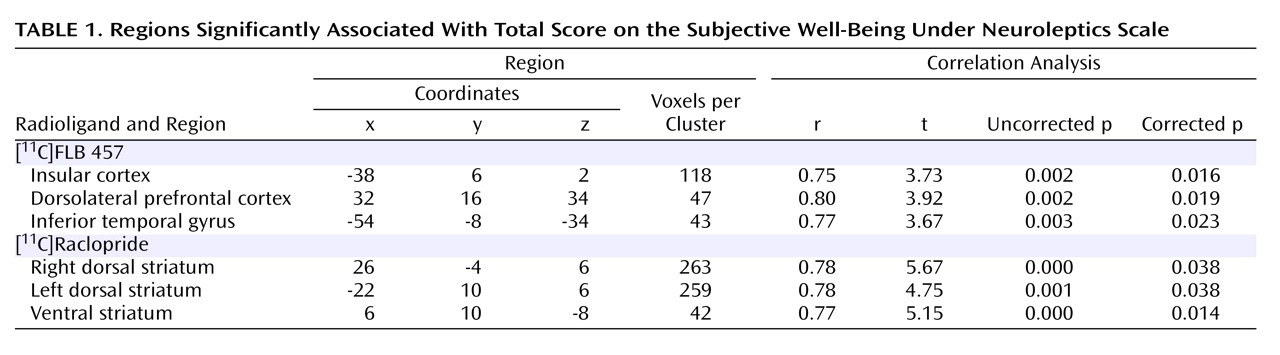
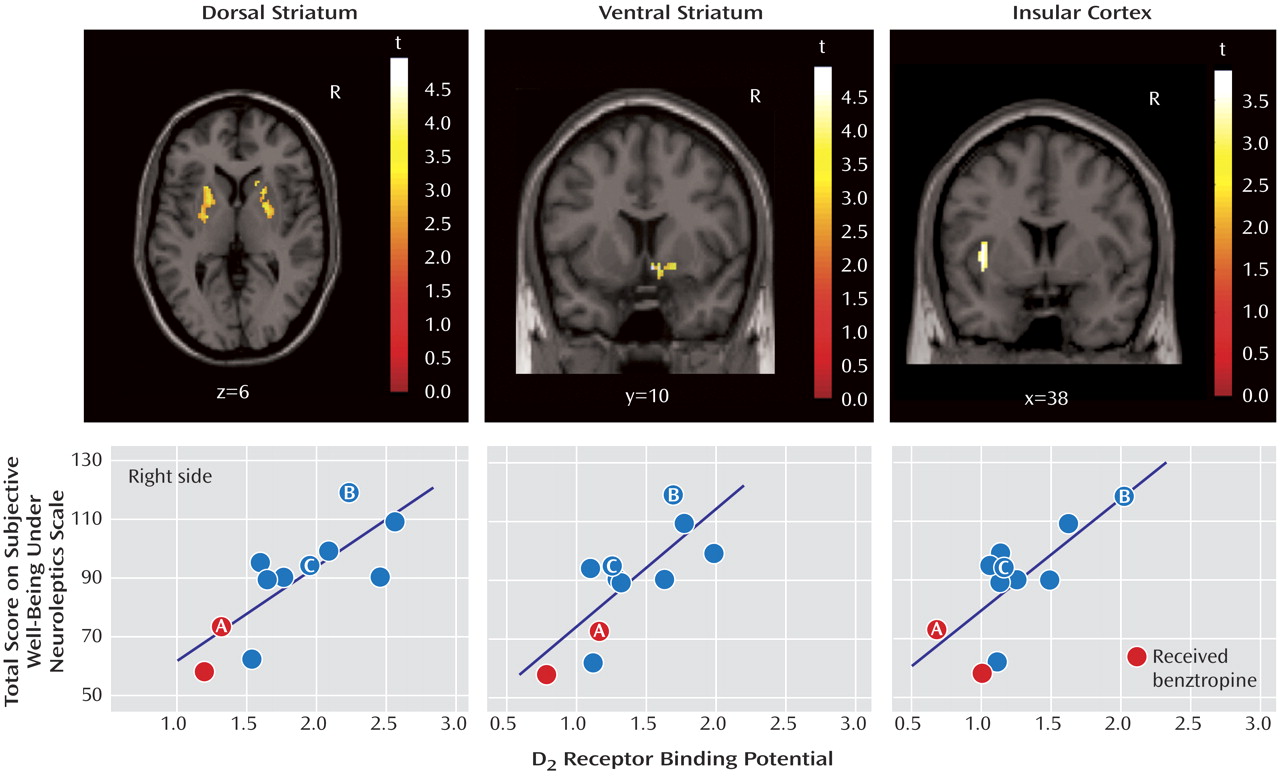
Since occupancy is a derived measure, i.e., it is obtained after correcting individual measured binding potential for estimated baseline levels, we wanted to examine if the raw binding potential data also correlated with Subjective Well-Being Under Neuroleptics scale score. The data showed that total score was significantly associated with striatal binding potential (r=0.63, p<0.03) and temporal binding potential (r=0.63, p=0.02) but not associated with frontal (r=0.34, p=0.30) or thalamic binding potentials (r=0.32, p=0.30). To obtain a finer-grained analysis of the subregions within the striatal and extrastriatal regions that correlated with Subjective Well-Being Under Neuroleptics score, we examined the correlation between voxel-based binding potential maps and score. Within the striatal region, we found that the total score on the Subjective Well-Being Under Neuroleptics scale was positively and significantly correlated with both the dorsal (right and left) regions (r=0.78, p=0.004 and r=0.78, p=0.004, respectively) and the ventral regions (r=0.77, p=0.005) of the striatum. Within the extrastriatal region, we found a strong correlation between insular cortex binding potential and score (r=0.75, p=0.007). The higher the binding potential in the insular cortex (i.e., lower occupancy by the antipsychotic drug), the higher the well-being. Smaller regions in the right dorsolateral prefrontal cortex (r=0.80, p=0.03) and the left temporal pole (r=0.77 p=0.005) also showed significant relationships (
Table 1 and
Figure 3 ).
Discussion
The study provides the first insight into the relationship between the patients’ subjective experience with antipsychotics and blockade of the dopamine D 2 receptors in different brain regions. Higher dopamine D 2 receptor occupancy in the striatal (dorsal and ventral), temporal, and insular regions was negatively associated with subjective experience when patients were receiving stable doses of antipsychotic medication. The robustness of this finding is supported by the fact that the same association is observed with both drugs under study (risperidone and olanzapine) using two methods of analysis (region-of-interest and voxel-based statistics) and two different dopamine receptor measures (observed binding potential values and age- and sex-corrected occupancy values).
The limitations of the present study are discussed to provide a context for further discussion. First, the sample size in the study was small. However, previous data that investigated the correlations between either AMPT or antipsychotic-induced dysphoria ranged from r=0.53
(23) to r=0.82
(7) . Using the standard 80% power and the conventional reliability to reject the null hypothesis (i.e., p<0.05), our study’s size was adequate to detect a significant correlation between those ranges. Finally, despite the small size we found converging results with several different methods, thereby enhancing the internal consistency of our findings. Limitations also exist in our estimates of D
2 receptor occupancies. To estimate binding potential for [
11 C]FLB 457 we used the Simplified Reference Tissue Model (SRTM), a model that assumes that the cerebellum has no specific binding
(22) . However, it has been shown that there may be a small, but measurable, signal arising from D
2 /D
3 receptor binding by [
11 C]FLB 457 in the cerebellum. It is estimated that the error (a potential underestimation of occupancies) induced by a displaceable [
11 C]FLB 457 signal in the cerebellum would range from 2%–5% for true occupancies in the range of 70%–90%
(24) . In addition, it has been shown
(25 –
27) that binding potential for dopamine D
2 /D
3 receptors in the thalamus is 8%–25% lower in patients with schizophrenia as compared with healthy volunteers. These differences in baseline could lead to a potential overestimation of occupancies that was estimated to range from 5% (SD=0.7) to 15% (SD=2.21). However, since such a group baseline difference by itself does not change the relative order of occupancies, it does not alter the associations with Subjective Well-Being Under Neuroleptics scale scores reported herein. While this simulation gives some reassurance, only a direct study measuring the baseline in individual patients can conclusively rule out the contributions of such a bias. In addition, the fact that we found a concordant association when the analysis involved the patient’s own measured binding potential and subjective experiences provides additional reassurance that this is not a false positive finding. Finally, we only investigated atypical antipsychotics, while typical antipsychotics have been associated with worse subjective experiences
(19) .
In the present work we replicated studies by de Haan and coworkers who reported an association between striatal D
2 blockade and Subjective Well-Being Under Neuroleptics score using SPECT
(8,
9,
23) . The present results are also consistent with the studies that have shown that disruption of the dopamine function produces dysphoria, decreases happiness, and provokes tiredness in healthy volunteers
(6,
28) and in drug-free schizophrenic patients
(7) . We extend those initial findings by showing that striatal blockade of the D
2 receptors was associated with impaired mental functioning and temporal blockade with altered emotional regulation. This is not surprising since the dopamine action has been associated with cognition, motivation, emotion, and action
(12) . What is also new in the present study is the fact that the ventral portion of the striatum, as well as the insular cortex (which is also part of the limbic system)
(29), were shown to have a specific association with Subjective Well-Being Under Neuroleptics score. It has traditionally been assumed that the same mechanism (D
2 blockade) that leads to the motor side effects may lead to the dysphoric subjective experiences reported by patients. The motor side effects of antipsychotics are typically associated with the dorsal region of the striatum
(30) . We found that the dysphoric effects correlate with not only the dorsal regions but also the ventral striatum and the insular cortex. These latter regions are considered parts of the “limbic” circuitry and can be plausibly linked to a role in subjective wellness. The ventral portion of the striatum (which is thought to be the analogue of the nucleus accumbens in animals) is directly implicated in reward and motivation
(15,
31) and is key to many of the nonmotor actions of antipsychotics in animals (i.e., conditioned avoidance response models)
(32) . It is well documented that increased dopamine release in the nucleus accumbens is associated with the rewarding effects accompanying the acute administration of most drugs of abuse
(33 –
35) . For example, euphoric responses elicited by amphetamine were inversely correlated with change in ventral striatum binding potential in healthy volunteers
(35) . In addition, the insular cortex, which has modest concentrations of dopamine D
2 receptors as measured in autoradiography studies in human brains
(36), has been implicated in the integration of internal states and has been associated with feelings of sadness
(37) and the subjective awareness of internal feelings
(38) . While the precise role of the dopamine receptors in the insula is not known, given its key role in integrating the extrapersonal stimuli and the internal milieu
(29), it is conceivable that altered transmission in this region may be experienced as an adverse subjective effect.
Taken together, the present data suggests that dopamine D
2 blockade, which is customarily related to antipsychotic efficacy and motor side effects, is also associated with the induction of dysphoria. However, there was no association between PANSS positive symptom and Subjective Well-Being Under Neuroleptics scores, suggesting that psychopathology and adverse subjective experiences are partially independent measures. In fact, less than 16% of the variance in Subjective Well-Being Under Neuroleptics can be explained by PANSS scores
(19) . Thus, clinicians face the paradox of using drugs that will improve symptoms but at the same time may induce dysphoria and hence reduce its adherence. This may be an intrinsic problem of D
2 -blocking antipsychotics. It may be the case that antipsychotics with novel mechanisms of action, e.g., partial D
2 agonists or “dopamine stabilizers”
(39) or drugs that avoid D
2 blockade altogether may be needed to avoid this dilemma. Of these options, only a partial agonist mechanism is currently clinically available (e.g., aripiprazole), and it would be interesting to see if this relationship is observed with aripiprazole.
In summary, a high proportion of patients taking antipsychotics report dysphoric experiences, and our study shows that this is associated with antipsychotic dopamine D
2 receptor blockade in different striatal and extrastriatal regions. Since this correlation was seen with atypical antipsychotics, and even in patients who do not show overt motor side effects, it seems that this negative effect may be an unavoidable consequence in many patients with high dopamine D
2 blockade. Given that these negative subjective effects may be related to the high discontinuation rates seen in clinical practice, future drug development may focus on antipsychotic drugs with a qualitatively different mechanism of action to avoid this effect.