Schizophrenia is not inherited because of a deficit in a single gene. Multiple genes appear to be involved in its inheritance, in addition to environmental factors. Because most analyses of genetic effects can be performed on only one gene at a time, gene hunting in schizophrenia becomes a risky business, fraught with uncertainties in research design and analytical conundrums. One way to determine the genetic architecture of schizophrenia is the endophenotype strategy illustrated by articles in this issue of the
Journal from Hall et al. and Gur et al. This strategy investigates the genetic basis of subclinical endophenotypes, each of which is hypothesized to reflect more directly the effect of a specific gene than the illness itself
(1) . The illness is then conceptualized as the product of the interaction of multiple genetically influenced endophenotypes, along with environmental factors.
Of course, the ultimate endophenotypes are perturbed levels of specific proteins or gene expression induced by inherited DNA sequence variations. This level of analysis is not yet possible in schizophrenia. Therefore, investigators turn to neurophysiological and neurocognitive measures that would seem to reflect more elementary aspects of the biology of the illness than the clinical features themselves. For example, poor processing of simple sensory information, measured electrophysiologically or neurocognitively, might be a more discrete reflection of a genetically determined brain dysfunction than a complex symptom such as persecutory delusions. Thus, Hall and colleagues examined a set of well-known electrophysiological abnormalities that have been associated with schizophrenia, and Gur and associates chose a neurocognitive battery that has previously shown deficits in neurocognition in schizophrenia. Some assumptions of endophenotype strategies have been challenged
(2) . Endophenotypes may not be as simple as one would hope. They often have their own complex neurobiological substrates, as revealed by functional brain imaging and model organism studies, and thus they may not be more tightly linked to genes than clinical phenotypes themselves.
Because there is no proven strategy to select brain dysfunctions that are linked to genes, investigators perform several tests to determine their relationship to the genetic transmission of schizophrenia. Here, two different methods were chosen. Hall et al. examined heritability in monozygotic and dizygotic twins, including twins concordant and discordant for the illness itself. This analysis of heritability is based on the hypothesis that monozygotic twins share all genes, because they come from one fertilized egg, and dizygotic twins share half their genes, like all siblings, because they come from two different eggs. Because a father contributes only one of two chromosomes and thus one of two copies of a gene to each sperm cell, siblings by chance will have only a 50% chance of getting the same gene copy from their father. The same odds apply for the distribution of gene copies from the mother. To the extent that a deficit in an endophenotype is genetically influenced, it should be on average only 50% similar in the dizygotic twins and 100% similar in monozygotic twins. Hall et al. used a sophisticated analysis to determine the actual fit of the data with this model, with other sources of variance accounted for by environmental factors. They also determined to what extent the similarity in endophenotypes resembled the sharing of risk for schizophrenia. They found that deficits in these electrophysiological measures of sensory processing were transmitted genetically and that the genetic transmission of the deficits was correlated with the genetic risk for schizophrenia.
The discordant monozygotic twins, one with schizophrenia and one without, are especially informative in the study, because of the presumption that the failure of one twin to develop illness means that some environmental factor present in the ill twin has combined with genetic factors present in both twins to produce the illness. Any abnormality measured as an endophenotype in the clinically well twin would then be presumed to be reflective of the genetic aspects of the illness, isolated from the environmental determinants
(3) . In fact, the offspring of the clinically normal monozygotic co-twin are at the same high risk of developing schizophrenia as the offspring of the ill twin
(4) . Similarly, neurological findings and neuropsychological performance frequently deviate from normal in the clinically ill and clinically well discordant twins
(5,
6) . An unanswered question is whether or not these genetically transmitted deficits, which are thought to carry risk for illness in some individuals, also carry adaptive advantages, such as optimizing vigilance in the other members of the family, who have the deficits without the illness
(7) .
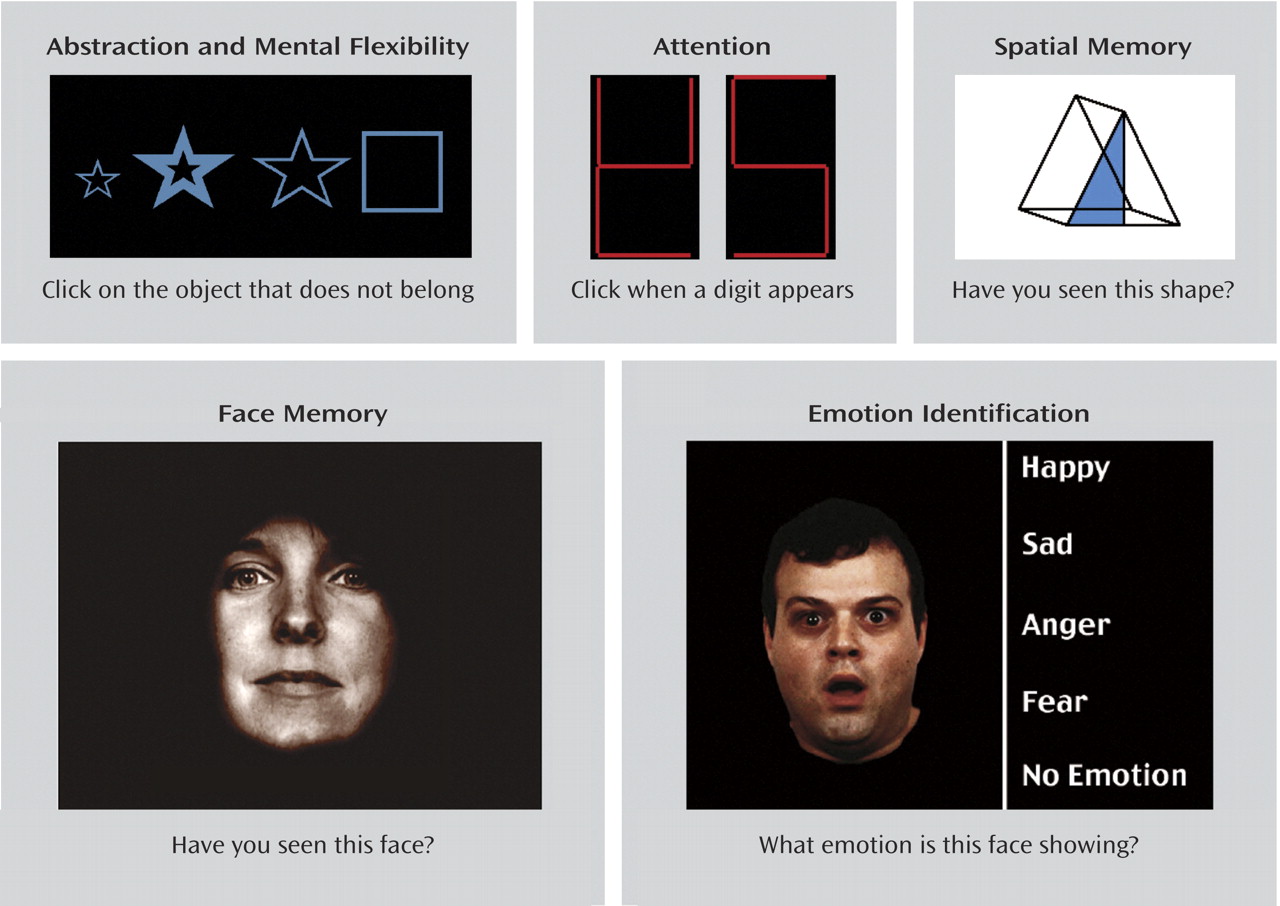
The electrophysiological measures used by Hall et al. have the advantage that they represent simple neurobiological functions, but deficits in responses to a series of clicks and tones do not have an immediate applicability to patients’ problems in functioning in the real world. Gur and her colleagues used a set of cognitive tests that are felt to be more closely related to real-life functioning; examples are shown in
Figure 1 .
Performance on these tests of perception, attention, social cognition, and memory has been linked to psychosocial dysfunctions in schizophrenia, such as employability and sustainability of interpersonal relationships. Instead of twins, this analysis relied on multigenerational sibships, in which schizophrenia is present in multiple siblings in one generation, as well as members of another generation, such as parents and uncles and aunts. The genetic analysis is similar in principle to the analysis performed by Hall et al. in the twins. Siblings are expected to share one-half of their genes with the other siblings, as well as half their genes with their parents. Uncles and aunts would be expected to share one-quarter of their genes. The extent to which performance on the neurocognitive tests follows this pattern determines heritability. The exception is those members of the family who have schizophrenia. If their illness is caused by the same genetic variants, then all the affected members of the family should have these variants.
Gur and colleagues found heritable deficits across a number of domains, including memory and spatial and emotional processes. They took into account not only accuracy on the tests, but also speed, because many persons with schizophrenia can manage to perform a task accurately, presumably by using more effort than other persons, but then it takes them longer to perform the task. Looking at speed as a criterion, the investigators found additional deficits in abstraction, face memory, and attention.
Whether there are links between the physiological deficits examined by Hall et al. and the neurocognitive deficits found by Gur et al. is a question that we are attempting to answer in a large genetic collaboration that examines both types of deficits in families with schizophrenia
(8) . Establishing heritability of endophenotypes and schizophrenia is crucial, but still only a first step in dissecting the genetic basis of the schizophrenia. The next step is to identify actual genes and genetic variations that contribute to the heritable fraction of the endophenotypes and to the genetic transmission of schizophrenia itself.