Morbidity and mortality are high in heroin dependence. Both can be reduced with methadone or buprenorphine
(1,
2), with efficacy of methadone maintenance treatment being best documented
(3 –
6) . Improving access to treatment therefore remains the most pressing issue in addressing the needs of heroin-dependent subjects. The utility of methadone maintenance therapy is, however, partly limited by its potential for overdose death, primarily following diversion and uncontrolled use
(7,
8) . Buprenorphine, a semisynthetic long-acting opioid, offers a decreased overdose risk compared to methadone because of its partial agonist properties at μ-opioid sites
(9) . This is supported by observational data
(10) . Accordingly, buprenorphine but not methadone is approved for office-based use in the United States. A shift to buprenorphine-based approaches therefore has a potential to reduce methadone overdose death and improve access to treatment. In fact, it has recently been proposed that buprenorphine should replace methadone as a treatment for heroin dependence
(11) .
It is, however, unclear whether this conclusion is justified. The partial agonism of buprenorphine is also commonly thought to limit its therapeutic efficacy, with maximal approved doses thought to be approximately equivalent to a 70 mg/day dose of methadone, whereas optimal doses of methadone average around 100 mg/day, and some patients require up to 140 mg/day
(12) . Given the high mortality of untreated heroin dependence, a superior efficacy of methadone maintenance therapy could outweigh any advantages of buprenorphine-based approaches. Available data seem insufficient to guide therapeutic choices. A recent meta-analysis concluded that the two treatments might be equally efficacious but reflected a considerable heterogeneity of underlying studies
(1), with some clearly favoring methadone
(13), while others found the compounds largely equivalent
(14,
15) . Most available comparative studies employed slow induction, fixed doses, and/or relatively low doses of both compounds and achieved outcomes that do not compare favorably with what can optimally be achieved with the respective compound
(16,
17) .
Currently, local therapeutic traditions, regulatory restrictions, and chance remain the major determinants of treatment for the individual patient. In attempting to address this, we note that the choice is not likely between each of the compounds in isolation. A stepped adaptive strategy employing both compounds may be advantageous to either drug alone in achieving an optimal balance between safety, accessibility, and efficacy
(17,
18) . In this strategy, all patients would be started taking buprenorphine, which can be escalated to the maximum approved dose of 32 mg/day. If the clinical effect remains insufficient at this dose, the patient can be transferred to methadone, which can be further escalated. With this, all patients who need methadone would ultimately receive it, but those who do not can be successfully treated without it. This approximates a proposed stepped approach to treatment of alcohol dependence
(19) .
Here, we evaluated a stepped strategy as outlined above, using a preparation of buprenorphine/naloxone (provided by Reckitt-Benckiser) that was approved for office-based treatment in the United States
(20,
21) . We compared stepped therapy to high-quality methadone maintenance therapy and also used the adaptive design of the stepped arm to evaluate whether a significant proportion of the patients would be successfully maintained with buprenorphine/naloxone without progressing to methadone and whether specific patient characteristics, e.g., severity, would predict the need to ultimately receive methadone.
Method
Overall Design, Recruitment, and Inclusion/Exclusion Criteria
The study is registered at http://www.clinicaltrials.gov and was approved by the Karolinska Regional Ethics Committee (dnr. 373/03). Information was disseminated by word of mouth and leaflets at addiction medicine clinics in Stockholm and Uppsala, Sweden. A self-referral prescreening form (enclosed with the patient information) was requested from prospective subjects, together with laboratory results (liver enzyme tests and HIV status) obtained at the local clinics. For those likely to be eligible, a doctor’s appointment was scheduled, a screening evaluation carried out, and written informed consent obtained.
The predefined study group was 96 subjects (48 per arm, 64 in Stockholm, 32 in Uppsala, Sweden). The power was ≥0.80 for detecting a difference in the primary outcome, proportion surviving, of 0.25 at α=0.05 and ≥0.80 at α=0.05 for the secondary outcome, Addiction Severity Index problem severity, to detect a difference between the groups reflecting a modest effect size (Cohen’s d=0.4). For a one-sided test for noninferiority on the primary outcome, the power was ≥0.80 at α=0.05 with an irrelevance interval of 0.15 in the proportion surviving
(22) .
Inclusion criteria were DSM-IV
(23) heroin dependence >1 year, age >20 years, and acceptance of the stated treatment principles. Exclusion criteria were severe psychiatric illness (dementia or psychosis) compromising the patient’s ability to provide informed consent, other clinically significant psychiatric illness unless stable under treatment, severe medical condition, other medical condition unless stable under treatment, treatment with antiseizure drugs or disulfiram, pregnancy or intent to become pregnant, or ongoing nursing. There was no restriction on prior participation in maintenance treatment, but patients involuntarily discharged from a maintenance program within 3 months were not eligible.
Random Assignment, Blinding, and Delivery of Medication
Each patient received an inclusion code of 1 to 96, a priori allocated to methadone maintenance treatment or stepped treatment in a computer-generated random sequence (block size 8, balanced between study sites). Random assignment was by the research pharmacy, insulated from trial staff. During this phase, only the research pharmacist and deputy had access to codes.
Blinding was achieved using the individual patient packs containing four identical-looking sublingual tablets containing buprenorphine/naloxone or placebo, in combinations yielding the desired dose, and one capsule of the respective dose of methadone hydrochloride or its placebo. A double-blind 24-day induction phase provided uniform dose escalation and stabilization for both arms (methadone maintenance treatment: 10 days to reach 70 mg/day of methadone; stepped therapy: 2 days to reach 16 mg/day of buprenorphine/naloxone). To avoid precipitating withdrawal, buprenorphine/naloxone was given upon the appearance of withdrawal symptoms, ≥8 hours after the last heroin intake.
After induction, allocation codes were communicated to the sites. Based on the below criteria, the local trial leader or designee wrote orders for 2-week intervals, blinded for the patient. Patterned after a prior report
(14), “transitions” were possible in intervals of 2 weeks. A transition was a dose increase or, in subjects receiving 32 mg/day of buprenorphine/naloxone, switching to methadone. Criteria for transitions were the following—within the preceding 2 weeks: ≤2 missed visits, self-reported insufficient blockade of craving, self-reported withdrawal symptoms on nadir, or any urine sample positive for illicit opiates and no signs of overdosing (cognitive impairment, sedation, respiratory depression). Methadone maintenance treatment was allowed transitions in 10-mg increments to 120 mg/day. Stepped therapy allowed transitions, in 8-mg increments, to 32 mg/day. If this was insufficient, a rapid switch followed; patients received 50 mg/day of methadone the day after the last buprenorphine/naloxone dose, followed by 10-mg increases every second day to 90 mg/day. After this, the methadone maintenance therapy protocol above was followed.
Behavioral and Other Interventions and Assessments
Patients met with case managers at least weekly for counseling and to provide information for dose adjustments. A slip (self-reported drug intake or any positive urine sample) led to the progression of 1) a dose increase; 2) if insufficient (i.e., indicators of slip/relapse continued to occur), or the maximum dose had been reached, intensified counseling to two and then three times a week. When 4 weeks’ stability in treatment had been achieved, defined by all-negative urine tests, but no earlier than after 3 months, patients were allowed take-away doses for weekends. With additional completed 4 weeks of stability, take-away doses were dispensed twice weekly, and after an additional 4 weeks of stability, once weekly. In case of relapse, daily supervised administration resumed. Patients were withdrawn from the study if they were absent from scheduled visits for more than a week; verbally or physically threatened or abused staff or patients; dealt drugs; or engaged in illicit drug use which by the responsible physician was deemed to endanger medical safety, e.g., intake of repeated high doses of benzodiazepines.
Patients participated in group relapse prevention therapy
(24,
25) focused on triggers for craving and techniques to recognize these and develop skills to cope with them without relapsing. Therapy consisted of 12 weekly sessions, led by two nurse-practitioner level therapists with special training.
Supervised urine samples were obtained in conjunction with the initial assessment visit and during the study. Samples were analyzed by the SWEDAC-accredited Clinical Pharmacology Laboratory at Karolinska University Hospital. Screening was made with the EMIT kit (Beckman Coulter, Bromma, Sweden), with cutoff values for opiates of 300 ng/ml, central stimulants of 300 ng/ml, cannabinoids of 300 ng/ml, and benzodiazepines of 100 ng/ml. All screening-positive samples were validated and quantified using SWEDAC-accredited liquid chromatography–mass spectrometry. Cocaine was not analyzed because the use of this substance is extremely rare in clinical heroin populations in Sweden.
On inclusion, as well as after 3 and after 6 months, problem severity was assessed using the semistructured Addiction Severity Index
(26) . The severity ratings are interviewer estimates of the patient’s need for additional treatment in each area. The scales range from 0 (no treatment necessary) to 9 (treatment needed to intervene in life-threatening situation). Each rating is based upon the patient’s history of problem symptoms, the present condition, and subjective assessment of his or her treatment needs in a given area.
Outcomes and Statistical Analyses
The primary outcome, retention in treatment, was analyzed by using Cox proportional hazard regression, with age, duration of heroin use, and gender as covariates. The assumption of proportional hazards was tested by using the time-dependent covariate test because this has good power for detecting nonproportionality
(27) . Noninferiority for censored survival data are commonly tested with a single time point comparison
(28), and this approach was used, as described
(22) .
Analyses of secondary outcomes were planned for completers only because the natural history of heroin dependence violates central assumptions of most commonly used approaches to dropouts. Thus, last-observation-carried-forward analysis assumes a significant correlation between data yielded in treatment and missing observations following dropout, while random effects models assume that available data points are a random sample of all possible observations. In fact, heroin-dependent subjects typically drop out in relation to relapse, invalidating these assumptions.
A secondary outcome in completers, Addiction Severity Index severity ratings were analyzed with repeated measures analysis of variance (ANOVA), with treatment as a between-subjects and time as a within-subjects factor. The other secondary outcome in completers was the proportion of urine samples negative for illicit opiates. Because of holidays, other scheduled absences, etc., the total number of scheduled urine sampling time points in completers varied between 48 and 54, with the majority submitting 52 samples. To equalize this, the study period was divided into six 1-month blocks, within which the proportion of clean samples was calculated for each subject. Missing samples were treated as positive. The proportion of clean samples was analyzed using two-way ANOVA, with treatment as between-subjects and block number as the within-subjects factor. The same approach was used for benzodiazepines and tetrahydrocannabinol (THC). Very few subjects had any central stimulant use, and this parameter was not further analyzed.
Finally, within the stepped therapy arm, a logistic regression analysis was used to evaluate whether gender, age, or severity or duration of dependence would predict switching to methadone. All analyses used Statistica 7.0 (StatSoft, Tulsa, Okla.).
Results
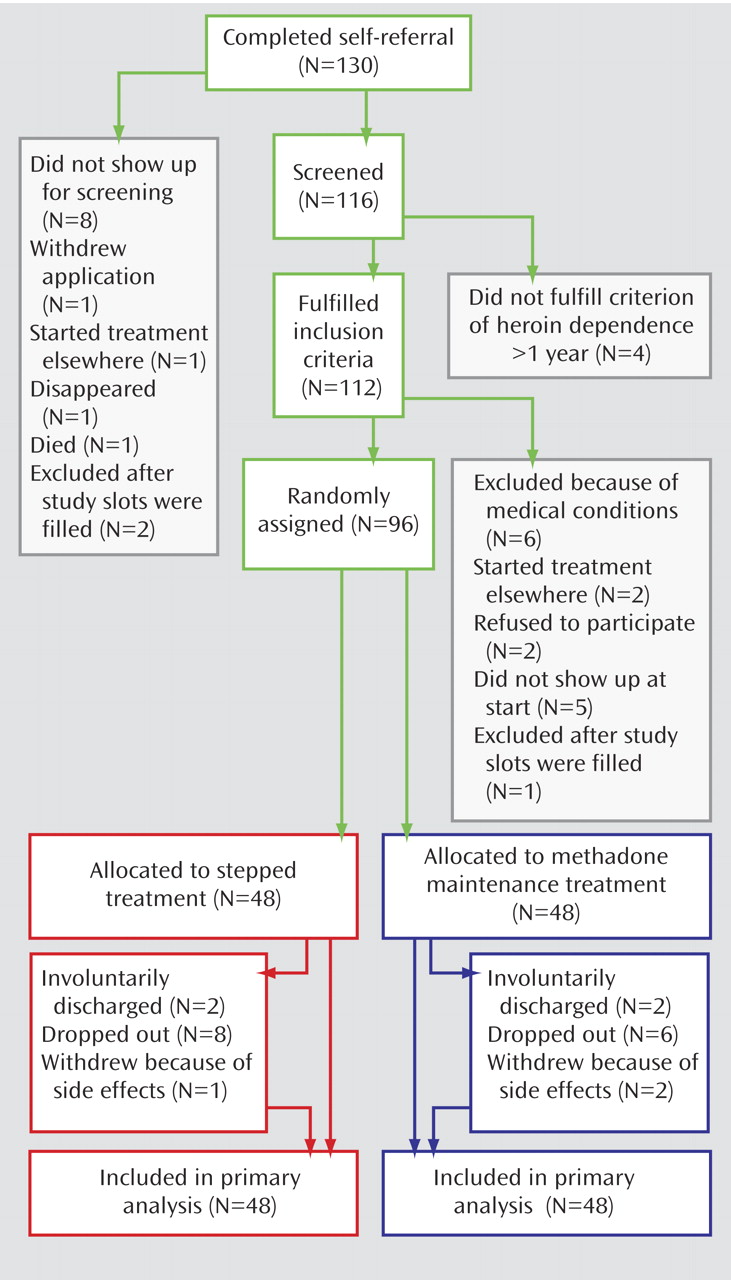
One hundred and thirty complete self-referrals were obtained: 116 subjects were screened, and 96 (83%) were randomly assigned from September 2004 to July 2005 (
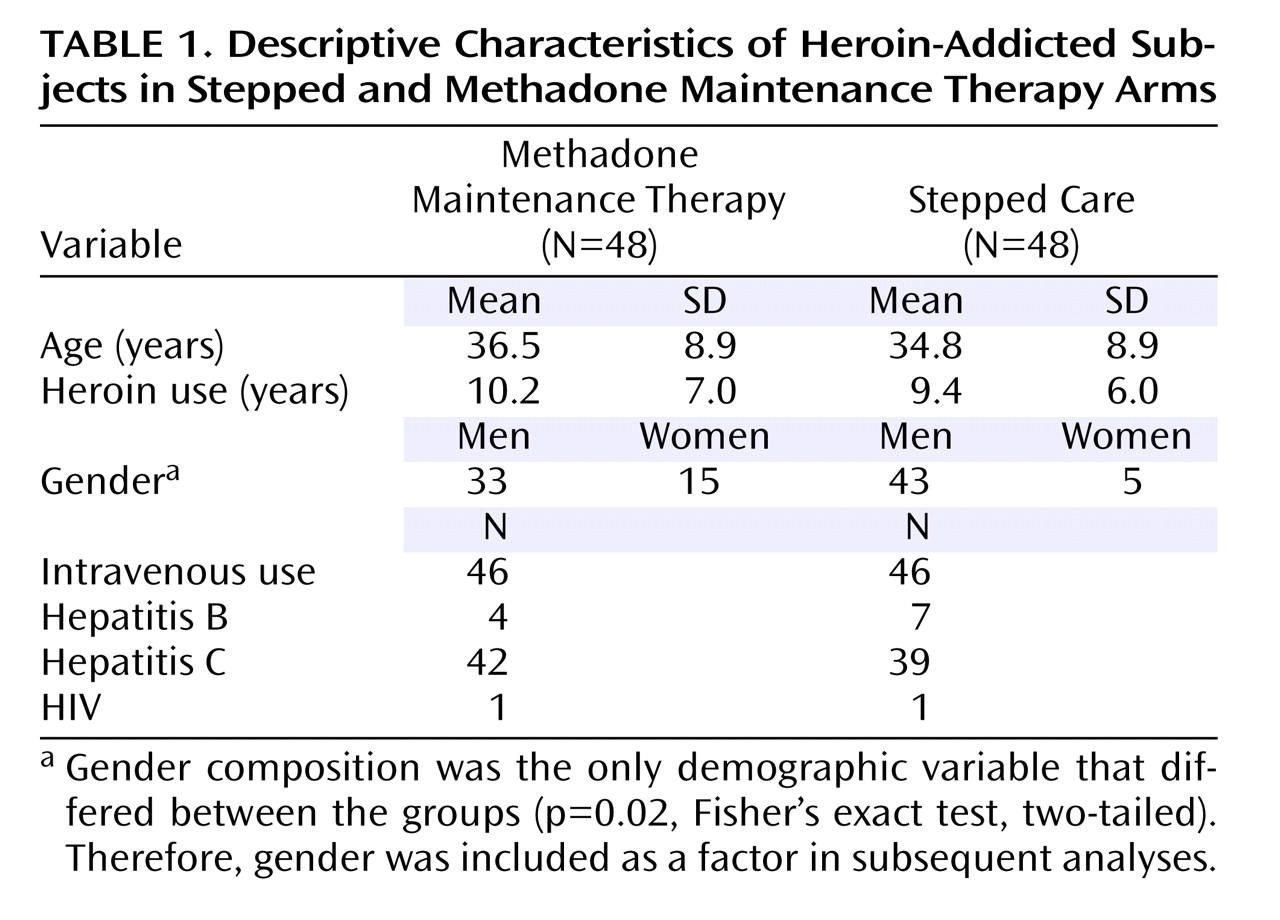
Figure 1 ). The two groups were similar in baseline characteristics, with the exception of gender distribution (
Table 1 ).
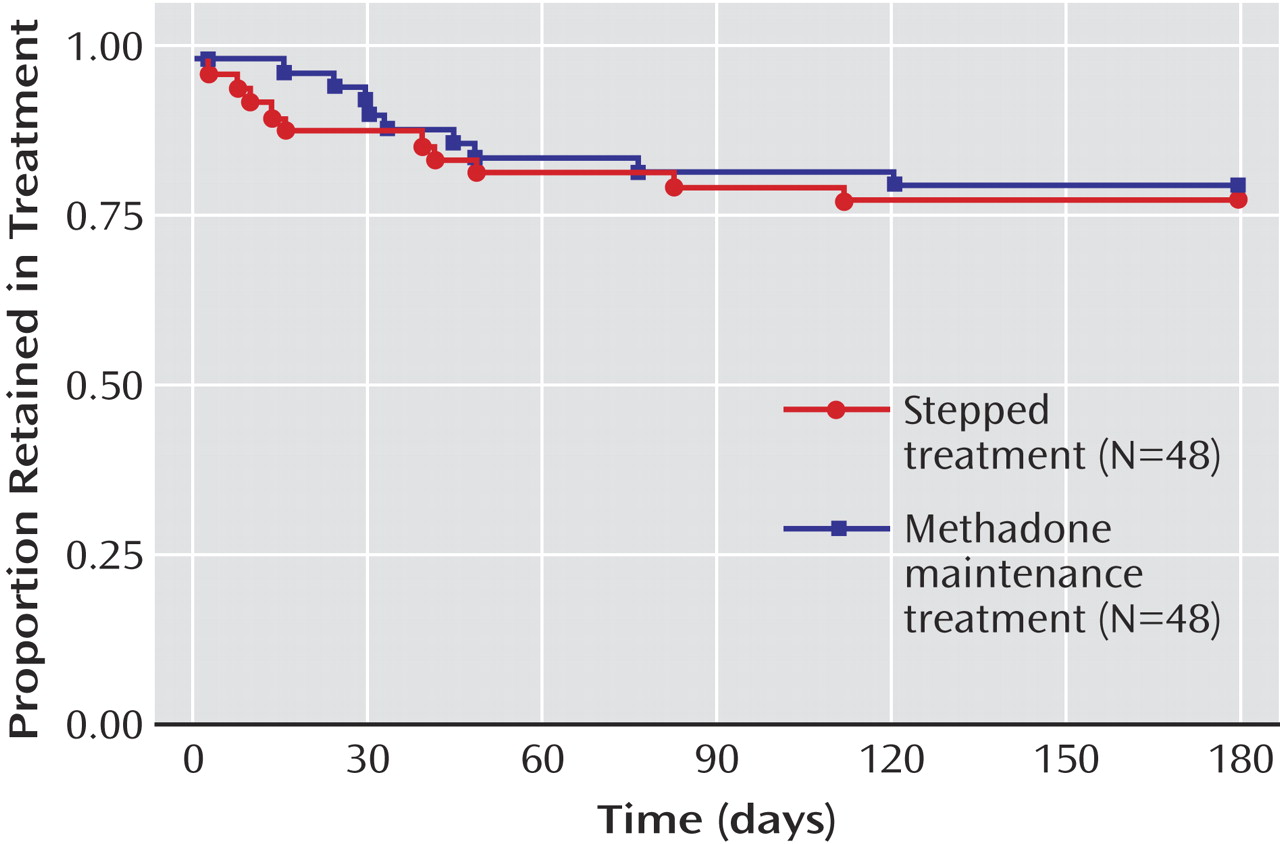
Overall retention was 78%. The results were virtually identical between arms (adjusted odds ratio=1.02, 95% CI=0.65–1.60;
Figure 2 ). A hypothesis of stepped therapy being inferior to methadone maintenance treatment with regard to the proportion of subjects retained could be rejected (z=–1.7, p<0.05) for δ≥0.15.
In stepped treatment, the final breakdown of the intention-to-treat group was 11 of 48 dropouts, 17 of 48 nonswitcher completers (final buprenorphine/naloxone dose: mean=29.6 mg/day, SD=4.7) and 20 of 48 switcher completers (final methadone dose: mean=111.0 mg/day, SD=11.7). By comparison, in the methadone maintenance treatment arm, 10 of 48 were dropouts, and 38 of 48 were completers (final methadone dose: mean=110.0 mg/day, SD=13.2).
Neither age, gender, duration of heroin use, nor baseline aggregate Addiction Severity Index severity ratings significantly predicted whether subjects in stepped therapy remained on buprenorphine/naloxone or switched to methadone (gender: p=0.48; age: p=0.34; years of heroin use: p=0.19; Addiction Severity Index score: p=0.93).
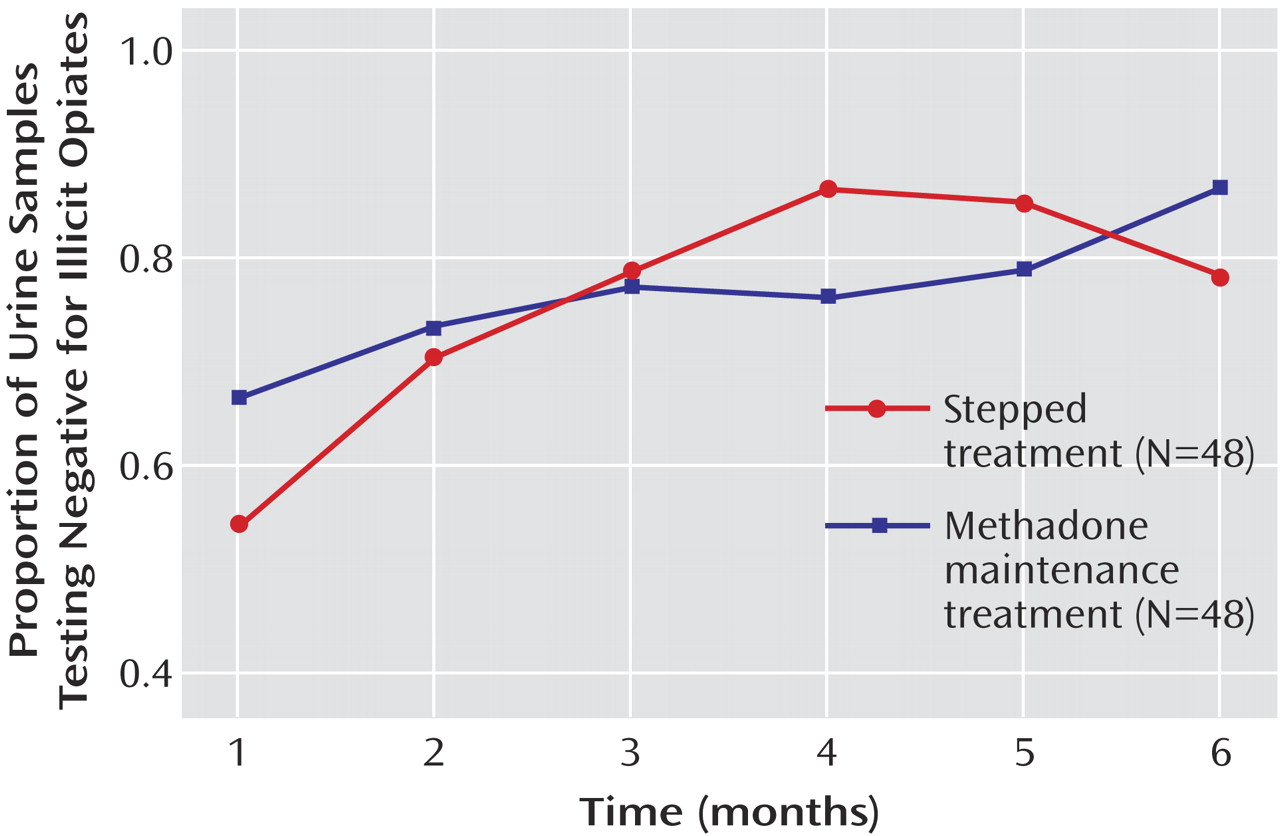
Overall, the proportion of urine samples free of illicit opiates over time increased (F=5.9, df=5, 438, p=0.00003). No difference between the two groups was found (p=0.87;
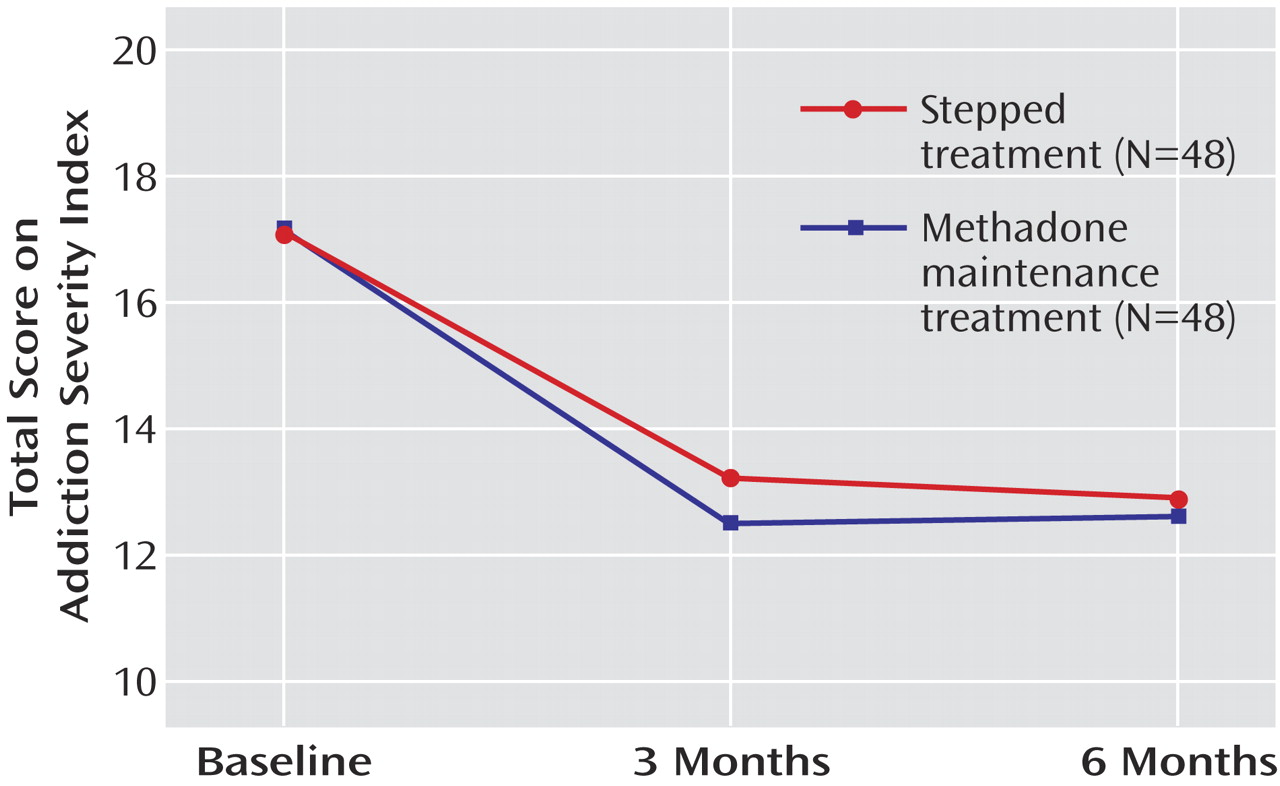
Figure 3 ). Similar results were obtained for benzodiazepines and THC (data not shown). Overall, problem severity as measured by the Addiction Severity Index decreased over time (F=17.1, df=2, 132, p<0.000001). No difference between the treatment arms was found (p=0.90;
Figure 4 ).
When Addiction Severity Index subscales were analyzed, reduction in total problem severity was mostly attributable to a reduction over time of drug-related problems (F=167.9, df=2, 132, p<0.000001). However, a significant reduction of problems related to occupation was also found already at this early stage of treatment (F=3.1, df=2, 132, p<0.05). Other Addiction Severity Index subscales were not significantly affected.
No serious adverse events were reported that were assessed as attributable to study medication. One patient was hospitalized after jumping off a balcony while alcohol intoxicated. Among nonserious adverse events, two patients from the methadone maintenance treatment group were withdrawn from the study because they developed lower leg edema, and one patient was withdrawn from the stepped arm because of respiratory difficulties. Two patients from each arm were involuntarily discharged after intoxication with illicit drugs. Other nonserious adverse events encountered, broken down by treatment arm, were methadone maintenance treatment: joint pain, muscle pain, elevated transaminase levels, vertigo, diarrhea, rigors, jaundice, abdominal pain, swollen lower legs; stepped therapy: diarrhea (two subjects), abdominal pain, appetite loss, insomnia, joint pain, vomiting, respiratory difficulties (while taking methadone), headache. Unless indicated otherwise, each adverse event was encountered in one patient.
Discussion
We report here for the first time outcomes for a stepped heroin dependence treatment that capitalizes on the advantageous properties of both buprenorphine/naloxone and methadone. Outcomes for stepped treatment and methadone maintenance treatment were virtually identical, and the noninferiority of stepped treatment was formally demonstrated on the primary outcome. Additional observations strengthen this conclusion. Virtually identical results between the two groups were also obtained on both secondary outcomes. Furthermore, when study results were stratified by participating center, virtually identical results between the two arms were again observed. The demonstration of noninferiority is particularly encouraging given that the comparator methadone maintenance therapy arm yielded a set of outcomes that compares favorably with any previously published studies of heroin dependence
(2), setting a high standard for the comparison.
The internal validity of our findings is likely high. Severity of both groups was similar. Gender distribution differed but did not contribute to outcome. The study was double blind during the sensitive initial month of treatment and single blind thereafter to allow truly flexible dosing. Despite close clinical monitoring, no observations indicated that patient blinding was unsuccessful. More important, despite the rapid switching methodology, no patients commented on transitioning from 32 mg/day of buprenorphine/naloxone to 50 mg/day of methadone. Thus, the single-blind design after the first month of treatment seems unlikely as a major limitation.
External validity of the results must be discussed in two contexts, that of patient selection and that of treatment setting. Patient selection is unlikely to pose limits on whether the findings can be generalized. In contrast to a previous study
(17) in which we targeted subjects early in their heroin career, the present inclusion criteria were broad, and the proportion of patients excluded was low. Accordingly, our population was representative of unselected intravenous heroin users in urban Sweden, with an average heroin use duration of 10 years. The study was carried out in the regular clinical setting of the two largest Swedish methadone maintenance programs. Together, this implies that many existing methadone programs, both in Europe and in the United States, could adopt the stepped strategy. By doing so, they would be able to successfully maintain a significant proportion of patients with buprenorphine/naloxone, gaining in safety without sacrificing efficacy. We believe that this is a goal worth achieving per se.
At the same time, it is clear that this alone would do little to broaden access to treatment. The major advantage of buprenorphine in this context is its relative safety for take-home dosing and its comparatively unrestricted availability in the United States. The relatively high intensity of psychosocial treatment and the stepped methadone rescue features of this study’s buprenorphine condition require that it initially be delivered in a setting similar to a traditional methadone maintenance program. Hence, it is clear that without changes to the treatment delivery system, the stepped strategy alone would do little to alleviate current treatment dissemination problems. Therefore, we believe that our results point to a need for changes in treatment delivery that could address this. We propose that this could be achieved by transforming existing methadone programs to specialized treatment centers that are not committed to a specific medication and that form nodes in a provider network to which office-based treatment providers are linked. By applying the stepped strategy evaluated here, specialized centers would efficiently identify patients who can be stabilized with buprenorphine/naloxone and ultimately referred out to office-based treatment. This closely approximates the system successfully implemented in Norway over recent years.
Several factors likely account for the overall excellent outcomes obtained here relative to other published controlled trials
(1,
2) . We used flexible dosing, and final doses were comparatively high. The final daily methadone dose was on average approximately 110 mg/day. Outcomes in methadone maintenance treatment are positively correlated with dose up to approximately this level
(29), but despite this, many clinical programs continue to use much lower doses, and few controlled studies have employed daily doses this high. For buprenorphine, a correlation between daily dose and outcome is less well established but does seem to be suggested by the meta-analysis of the buprenorphine literature
(1) . It is unclear why buprenorphine doses beyond 16 mg/day would improve outcomes, since this level produces 80%–95% μ-opioid receptor occupancy
(30) . An interesting mechanism is suggested by the recent observation that buprenorphine at higher doses is an agonist at nociceptin receptors
(9) . We have recently shown that this mechanism accounts for an ability of high buprenorphine doses to suppress self-administration of another drug of abuse, alcohol
(31) . Finally, the naloxone component of buprenorphine/naloxone could contribute to a need for higher doses if the naloxone were partially absorbed, although available studies suggest that the buprenorphine-only and the buprenorphine/naloxone preparations are equipotent
(20) .
Our study was not designed to evaluate the contribution of the behavioral treatment, but this has likely been key to the outcomes obtained. We combined the pharmacological treatment with behavioral methodology based on close monitoring of illicit drug use by supervised urine sample collection, reinforcement of treatment compliance, and relapse prevention group therapy—all methods known to improve treatment outcomes
(17,
32,
33) . It is important to note that this was done using available clinical resources and should be possible to implement in many clinical programs. Of note, we used an unusual combination of a low threshold for inclusion and a nonconfrontational approach on one hand, with a highly structured treatment program on the other. Finding a balance between patient autonomy and structure in the treatment of heroin dependence has been raised as a key factor in determining outcomes and remains controversial
(34) . The importance of and difficulties in finding this balance have recently been discussed, and our treatment model essentially follows the sequential progression from permissive to structured suggested
(16) . The marked decrease in use of illicit opiates over time and the decreased problem severity and improved occupational status suggest that with this approach, patients not only stayed in treatment, but in fact initiated a process of functional improvement. It is particularly encouraging to observe these benefits in the relatively limited time frame of a 6-month study because it has previously been shown that this type of benefit peaks only after about 4 years of methadone maintenance treatment
(16) .
In conclusion, to our knowledge, this is the first report of results obtained with a stepped care approach to heroin dependence that capitalizes on the advantageous properties of both buprenorphine/naloxone and methadone. The study shows that a significant proportion of patients who could be retained in treatment remained on buprenorphine/naloxone in such a stepped approach, presumably translating into major safety gains. The efficacy of this approach appears equal to the gold standard of optimally delivered methadone maintenance treatment. Together, these results imply the following balanced position in the current debate over the role of buprenorphine and methadone in the treatment of heroin dependence: buprenorphine should neither replace methadone, as recently suggested
(11), nor be viewed as a less effective alternative to methadone when the latter is not available. Instead, it should generally be used as the first-line treatment in heroin dependence, with provisions for rapid progression to methadone when needed.