In patients with schizophrenia, neurocognitive deficits have been established as an important symptom domain associated with long-term outcome. On average, these patients perform one to two standard deviations below healthy individuals on neurocognitive measures, such as those assessing attention, executive function, memory, and processing speed
(1,
2) . These deficits are clearly present at the first episode of illness
(3 –
5), even in antipsychotic-naive patients
(6,
7), and thus are not a deleterious effect of treatment. These deficits appear to be only marginally corrected with conventional antipsychotic agents in patients early in the course of psychotic illness, even when medication is administered at lower doses
(8 –
10) .
Treatment with atypical antipsychotics has been found, in various studies, to produce improvements in neurocognitive performance in schizophrenia
(3,
13 –
23 ; see also the meta-analyses in references
20,
24,
25 and early psychosis in references
8 –
10) . However, the comparator medication in many of these studies was a conventional antipsychotic administered at doses large enough to produce substantial extrapyramidal symptoms requiring treatment with anticholinergics, and both of these classes of medication are associated with impaired cognition
(20) . In previous studies, antipsychotic-naive patients with early psychosis receiving olanzapine
(8,
9) or risperidone
(10) demonstrated greater neurocognitive improvement than those receiving haloperidol, even when haloperidol was given in low doses. In this study, we compared the neurocognitive effects of olanzapine and risperidone and a third atypical antipsychotic, quetiapine. Our primary hypothesis was that the three agents would be equivalent in their effects on various neurocognitive measures.
Method
This was a 52-week randomized, double-blind, flexible-dose, multicenter study of patients early in the course of schizophrenia, schizoaffective disorder, or schizophreniform disorder assigned to treatment with olanzapine, quetiapine, or risperidone.
Study Population
Participants were recruited from inpatient, outpatient, and emergency department services for the evaluation and treatment of psychosis. The study was approved by the institutional review board at each site, and written informed consent was obtained from the patients or their legally authorized representatives. Consenting patients 16–40 years of age were eligible for the study if they met DSM-IV criteria for schizophrenia, schizophreniform disorder, or schizoaffective disorder. Patients had to be in the first episode of their psychotic illness and had to have been continuously ill for at least 1 month and no more than 60 months. Patients were excluded if a prior psychotic episode had remitted for 3 months or more or if they had prior antipsychotic drug treatment for more than 16 cumulative weeks. Several exceptions to these criteria were allowed on a case-by-case basis: the study included nine patients who had been ill for more than 60 months, seven who were over 40 years of age, and 16 who had taken antipsychotics for more than 16 weeks. All patients had a score ≥4 on at least one psychosis item in the Positive and Negative Syndrome Scale (PANSS; 27) and a score ≥4 (moderately ill) on the severity of illness item of the Clinical Global Impression scale (CGI) at the point of maximum severity of illness to date. Female participants of childbearing potential had to be using a medically acceptable form of contraception.
We excluded patients who did not speak English; had a history of mental retardation; were pregnant or nursing; had a serious, unstable medical illness; had a known allergy to one of the study medications; were at serious risk of suicide; or had participated in an investigational drug trial within 30 days before the first treatment visit.
Study Treatments
Patients were randomly assigned to treatment with olanzapine (2.5–20 mg/day), quetiapine (100–800 mg/day), or risperidone (0.5–4 mg/day). On days 1 and 2, each patient received one capsule of olanzapine (2.5 mg), quetiapine (100 mg), or risperidone (0.5 mg) in the evening. At the treating physician’s discretion, the dose could be increased by one capsule every other day—i.e., on days 3 and 4, one capsule in the morning and one in the evening; on days 5 and 6, one capsule in the morning and two in the evening; and so on, up to a maximum of four capsules twice daily.
Any previous antipsychotic therapy was tapered and discontinued during the first 2 weeks of double-blind treatment, and no subsequent use of an additional antipsychotic was permitted. Treatment with an adjunctive antidepressant or mood stabilizer during the first 8 weeks of treatment was not allowed unless approved by the project medical officer. Anticholinergic medications for acute extrapyramidal side effects were permitted for up to a total of 2 weeks over the course of the trial. Clinicians were encouraged to lower the dose of antipsychotic to relieve extrapyramidal side effects. Otherwise, adjunctive and concomitant medications could be used without restriction. This strategy kept the frequency of use for benzodiazepines, antidepressants, mood stabilizers, and anticholinergics below 5% during the study.
Assessments
The screening evaluation included a diagnostic interview (the Structured Clinical Interview for DSM-IV), medical history, physical examination, measurement of vital signs, and laboratory tests.
Training covering the study protocol and administration of all study evaluations was provided at an investigator meeting. Cognitive testers who could not attend the meeting and raters who joined the study after the investigator meeting participated in the same training using web-based materials, teleconferencing, and phone certification procedures.
Study visits occurred at baseline, at weekly intervals for the first 6 weeks, every other week for the next 6 weeks, and monthly thereafter. Neurocognitive assessments were conducted at baseline (up to 2 weeks after the start of treatment) and at weeks 12 and 52 (up to 2 weeks before or after the target date) or when the patient terminated the study if it was before week 52. A total of 224 patients completed neurocognitive assessments at baseline and 12 weeks, and 81 patients also completed them at 52 weeks. Cognitive testers were not blind to adverse event status and use of concomitant medications.
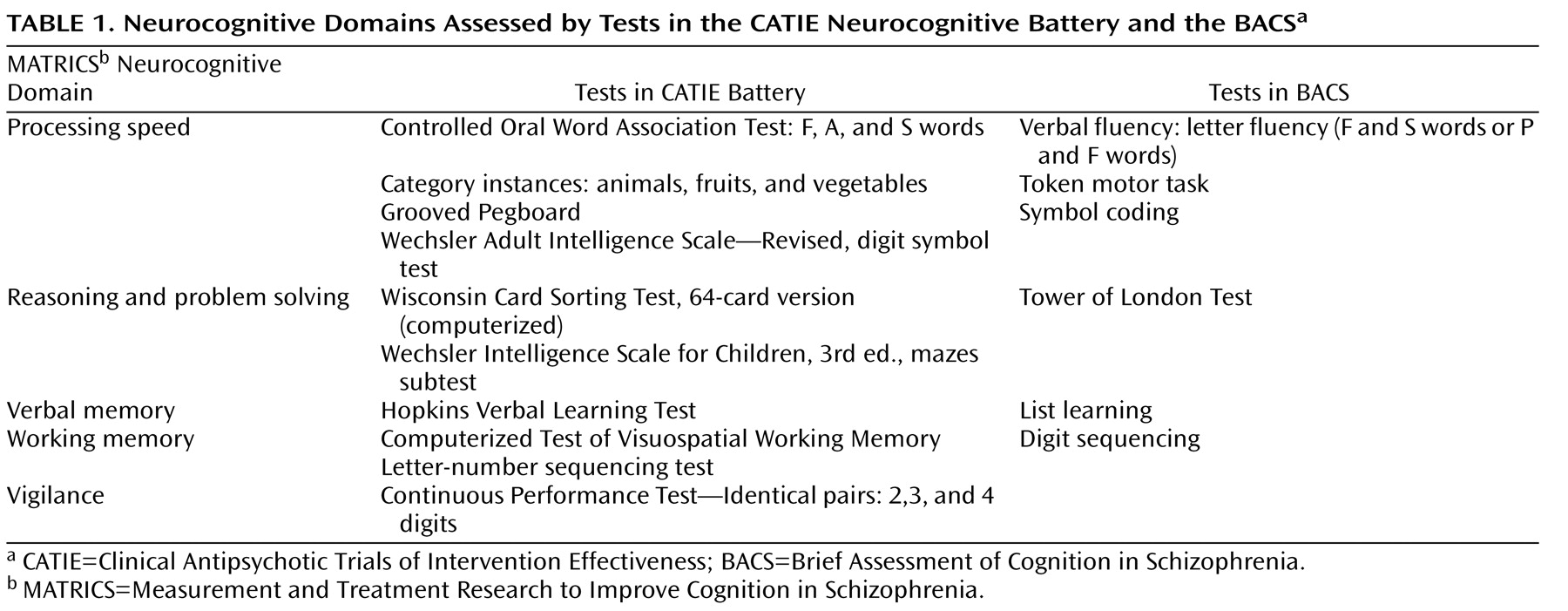
The primary neurocognitive outcome measure for this study was a composite score derived from the neurocognitive battery used in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). The performance of the 1,331 patients assessed at baseline is described in detail elsewhere
(28) . For the calculation of the CATIE battery composite score, all test measures were first converted to standardized z scores by setting the sample mean of each measure at baseline to zero and the standard deviation to 1. Summary scores for some tests were calculated by averaging z scores from individual measures: a Wisconsin Card Sorting Test score was calculated by averaging z scores for preservative errors and categories achieved; a Continuous Performance Test score was calculated by averaging the z score of
d -prime for the three different Continuous Performance Test conditions; and the Controlled Oral Word Association Test and category instances summary measures were averaged together to form one summary test score referred to as verbal fluency. For domains with more than one test, summary scores were determined by calculating the mean of the z scores for the measures that comprised the domain, then converting the mean to a z score with a mean of zero and a standard deviation of 1. This resulted in nine test summary scores and five domain scores corresponding to five of the seven domains in the Measurement and Treatment Research to Improve Cognition in Schizophrenia consensus battery
(29) . A domain-based composite score was defined as the average of the five domain summary scores for the CATIE neurocognitive battery.
An additional neurocognitive outcome measure was the composite score on the Brief Assessment of Cognition in Schizophrenia (BACS; 30, 31), a briefer set of tests designed to derive a composite score. With the BACS, we sought to determine its relative sensitivity to treatment-related cognitive changes compared with the larger CATIE battery; we also wanted to enable comparisons between this study and other trials using the BACS. The BACS, which takes approximately 35 minutes to administer, includes brief assessments of reasoning and problem solving, verbal fluency, processing speed, verbal memory, working memory, and motor functions. The BACS composite score was calculated by summing the z scores for each of the six measures, obtained by comparing each measure with a healthy comparison sample and dividing by the standard deviation of the healthy comparison sample
(30) . This composite score has high test-retest reliability in patients with schizophrenia and healthy comparison subjects (intraclass correlation coefficients >0.80)
(30) . Pearson correlations between the BACS composite score and CATIE neurocognitive composite score in this study were r=0.84 at baseline, r=0.86 at 12 weeks, and r=0.90 at 53 weeks (all p values <0.001).
Table 1 summarizes the neurocognitive domains assessed by the CATIE battery and the BACS.
Severity of psychopathology was measured with the PANSS and the CGI severity item. Functional outcome was measured with the standard patient interview from the Heinrichs-Carpenter Quality of Life Scale
(32), emphasizing the vocational and social outcome dimensions.
Statistical Analysis
Analyses of the neurocognitive variables were specified in a statistical analysis plan that was finalized before the blind was broken. Baseline measures of demographic and clinical characteristics were compared using two-sided Kruskal-Wallis tests for continuous variables and Fisher’s exact test for categorical variables. Separate general linear models provided least square means estimates for changes in cognitive scores from baseline to weeks 12 and 52 for each treatment group, adjusting for baseline and weeks of treatment in cases where neurocognitive testing was completed 1 to 2 weeks before or after the scheduled visit. The effect of group membership on neurocognitive change scores was tested using the F statistic from the model, followed by pairwise comparisons between treatment groups if overall treatment effect was significant. Pearson partial correlation coefficients were used to examine the potential linear relationships between treatment-related changes in the neurocognitive composite scores and treatment-related changes in the clinical outcome measures and the social and vocational subscale scores on the Heinrichs-Carpenter Quality of Life Scale from baseline to weeks 12 and 52 in each treatment group and in the cohort as a whole. These correlations were adjusted for baseline cognitive and clinical measures.
The primary analysis tested the overall treatment effect on CATIE neurocognitive battery composite scores from baseline to week 12 at the 0.05 significance level. All subsequent analyses on individual cognitive tests and between treatment groups were intended to expand our major finding and should be considered exploratory. Therefore, no p value adjustments were made for multiple comparisons.
Discussion
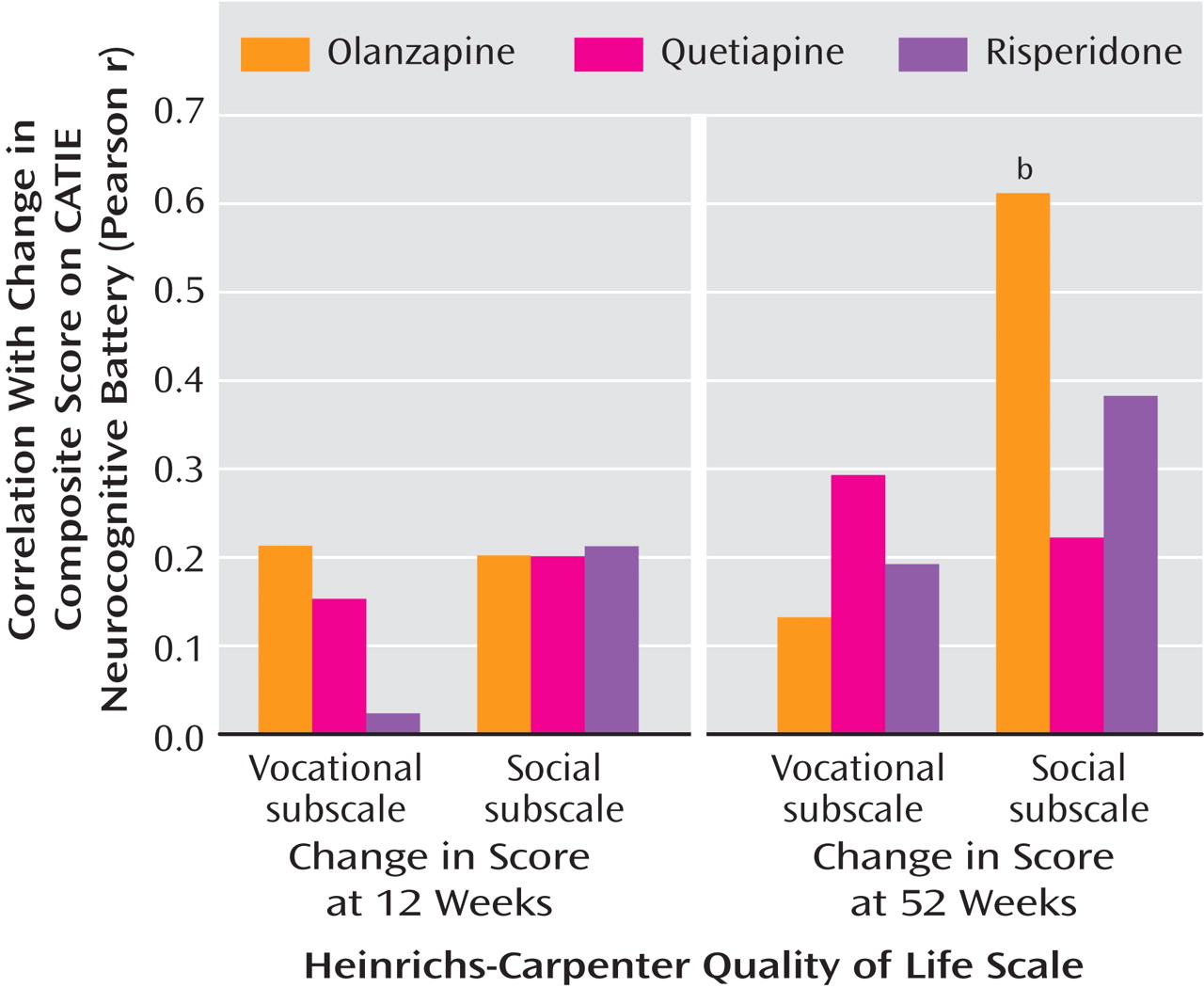
This is the first randomized, double-blind study comparing the neurocognitive effects of atypical antipsychotic agents in the treatment of patients early in the course of psychotic illness. Olanzapine, quetiapine, and risperidone produced modest but significant improvements in neurocognitive test performance. There were no significant differences between treatments in overall cognitive composite scores, which suggests that quetiapine may provide modest cognitive benefit to patients with early psychosis that is in line with that provided by olanzapine and risperidone. Improvement in the cognitive composite scores was significantly associated with improved social and occupational functioning as measured by the Heinrichs-Carpenter Quality of Life Scale. This result is the first direct evidence that antipsychotic treatment-related cognitive changes in patients with early psychosis may be clinically relevant for occupational and social functioning. However, interpretation of this relationship is tempered by analyses indicating that symptom change and baseline cognitive scores also predicted the variance in functional outcomes. Thus, cognitive improvement may be a part of a general treatment response that is associated with improved functional outcomes.
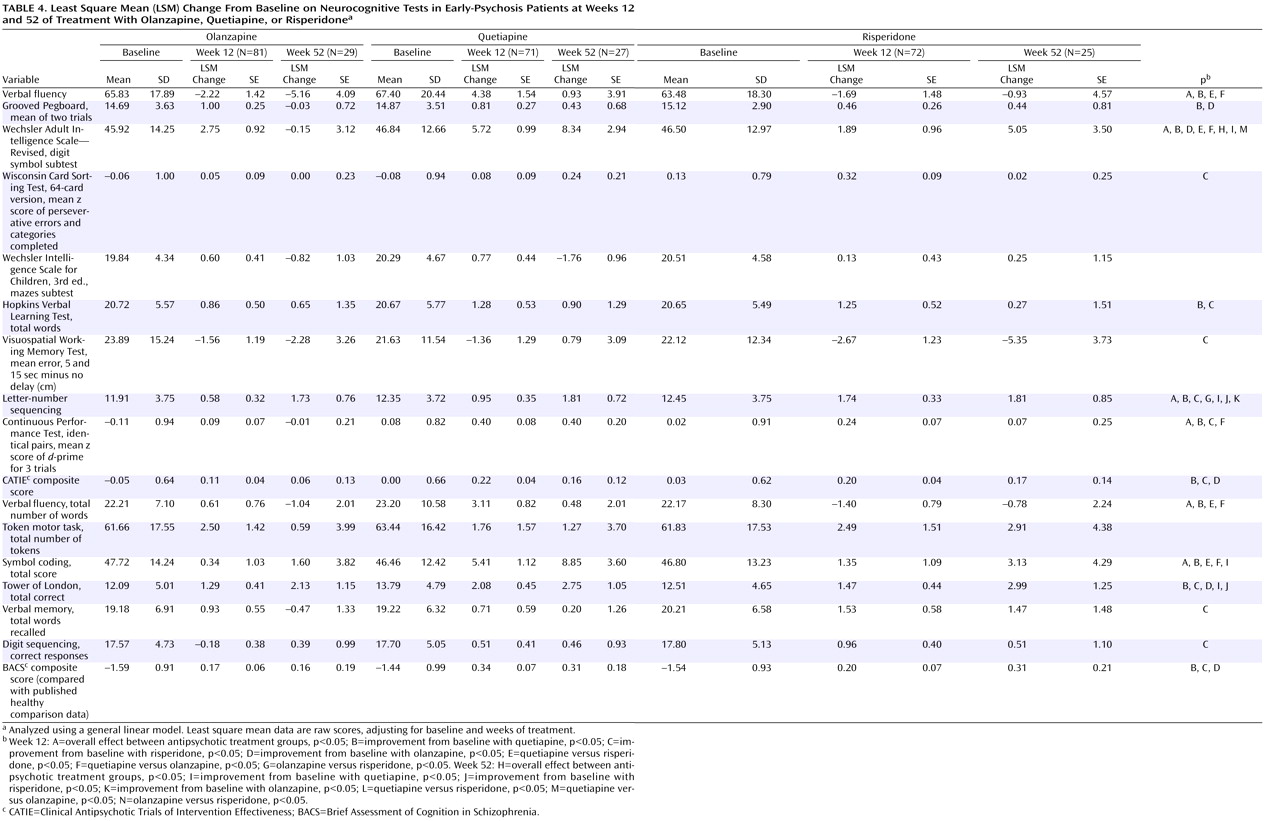
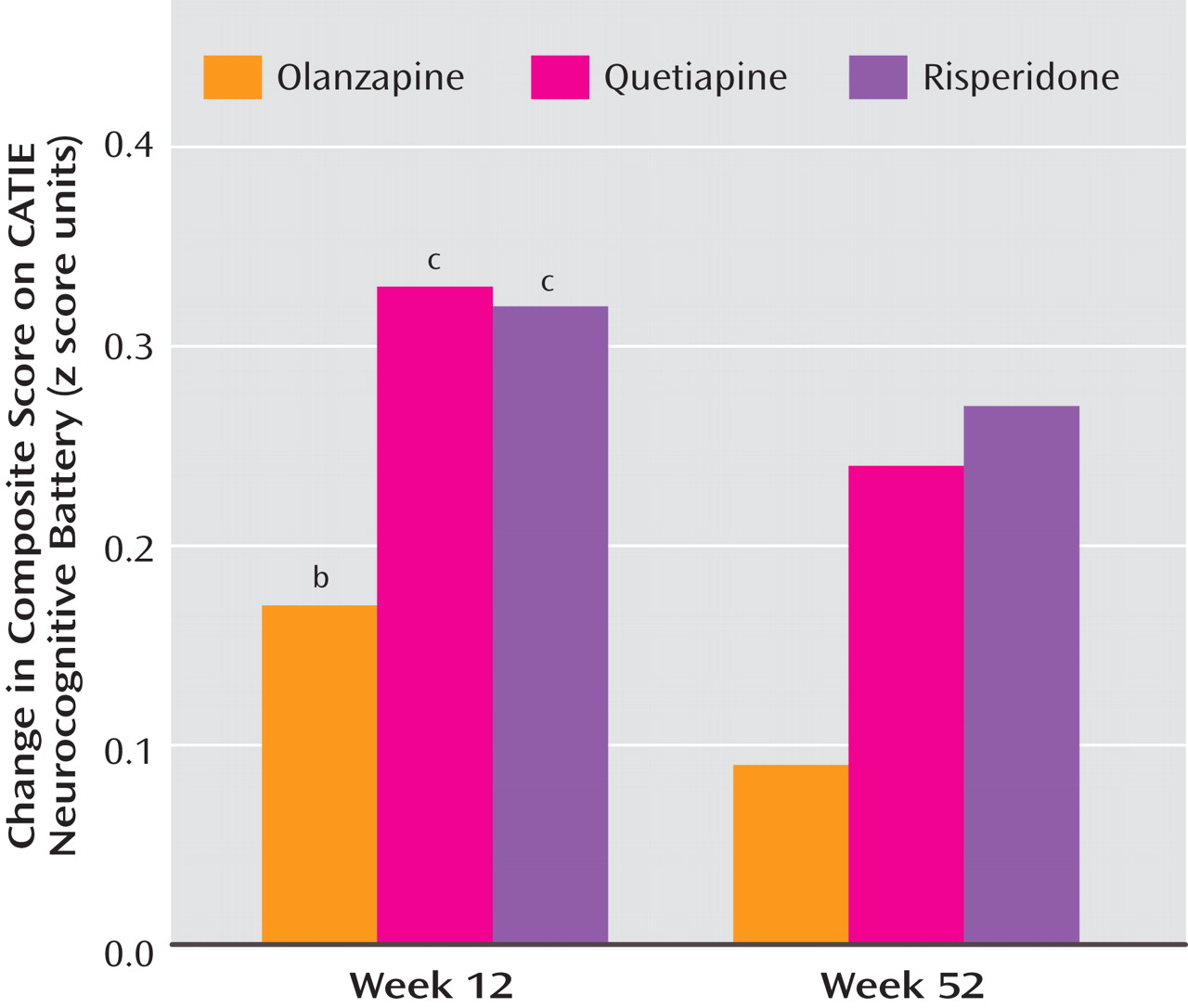
The magnitude of neurocognitive improvement for olanzapine and risperidone in this study is slightly less than that previously reported in comparisons with conventional antipsychotics in early-psychosis patients
(8 –
10) . In earlier studies that used doses similar to those we used in this study
(8,
9), the magnitude of the effect of olanzapine on neurocognitive composite scores was 0.36 at 12 weeks and 0.56 at 52 weeks; in this study, olanzapine’s effect was weaker, with effect sizes of 0.17 and 0.09 at 12 and 52 weeks, respectively. The magnitude of the effect of risperidone on neurocognition in an earlier study
(10) was 0.4 at 12 weeks, whereas in this study, effect sizes for risperidone were 0.32 and 0.27 at 12 and 52 weeks, respectively.
The reason for the lesser effect of olanzapine and risperidone on neurocognition in this study compared with previous studies is not entirely clear. These differences in magnitude are small and could be due to random factors or to methodological advances in the current study that may have served to refine the estimate of the magnitude of cognitive treatment effects, such as the inclusion of alternate verbal memory tests, which is likely to yield lower estimates of improvement because of repeated test exposure
(8 –
10) . Another possible factor is prior exposure to antipsychotics. Patients who are antipsychotic-naive when beginning treatment have been shown to obtain particularly large benefits from treatment with atypical antipsychotics
(9) . While about three-quarters of the patients in this study and in the earlier olanzapine study had briefly received treatment with antipsychotics prior to randomization, in this more recent study the medications received previously were more likely to have been atypical antipsychotics, particularly olanzapine or risperidone. Patients who were randomly assigned to the same medication that they were receiving at or before baseline may have had a reduced neurocognitive response to further treatment
(36) . Moreover, patients were given a 2-week window after treatment initiation to complete baseline neurocognitive testing. Thus, very early cognitive effects of antipsychotic treatment might have occurred in some of these patients, which may have minimized measurements of cognitive treatment response.
The significant benefit of quetiapine treatment on neurocognitive measures in patients with early psychosis is a new observation. Previous work has suggested that olanzapine
(8,
9) and risperidone
(10) provide greater cognitive benefit than low doses of haloperidol in patients with early psychosis. Our findings in this study suggest that the effect of quetiapine on cognition may be as beneficial as that of olanzapine or risperidone, and thus this agent may be another evidence-based alternative for clinicians who focus on cognitive outcomes. However, results from a recent study of 240 schizophrenia patients with stable symptoms treated with donepezil or placebo over a 12-week period suggest that the amount of cognitive change we report here is consistent with what may be expected from practice effects and placebo effects (unpublished 2004 study of R. Keefe et al.). This series of results raises the question of whether even low doses of haloperidol have a deleterious impact on cognition and whether the cognitive benefit of atypical antipsychotics derives from their reduced adverse effects rather than procognitive effects. It is noteworthy, however, that this negative effect may not occur with the conventional antipsychotic perphenazine, whose cognitive effects were similar to those of atypical antipsychotics in the CATIE schizophrenia trial (37).
Although much of our results stem from exploratory analyses with a large number of outcome measures not corrected for multiple comparisons, the pattern of results raises the possibility that quetiapine may have particular benefit on tests of verbal fluency and coding, in both the processing speed domain
(38) and vigilance. These data support previous findings
(16,
19,
22) as well as conclusions from a meta-analysis suggesting that quetiapine has a particularly beneficial impact on verbal fluency and vigilance
(24) . Perhaps quetiapine’s lack of appreciable affinity for muscarinic cholinergic receptors
(39), which minimizes anticholinergic effects, and its fast dissociation from striatal dopamine D
2 receptors
(40), which minimizes potential adverse effects on frontostriatal systems (including reduced thalamocortical drive), allow for more efficient processing speed.
The course of cognitive improvement with atypical antipsychotics is controversial. While some long-term studies have reported increasing cognitive improvement over time
(9,
41), others have not
(42) . Furthermore, the additional cognitive benefit over time may depend on significant patient attrition
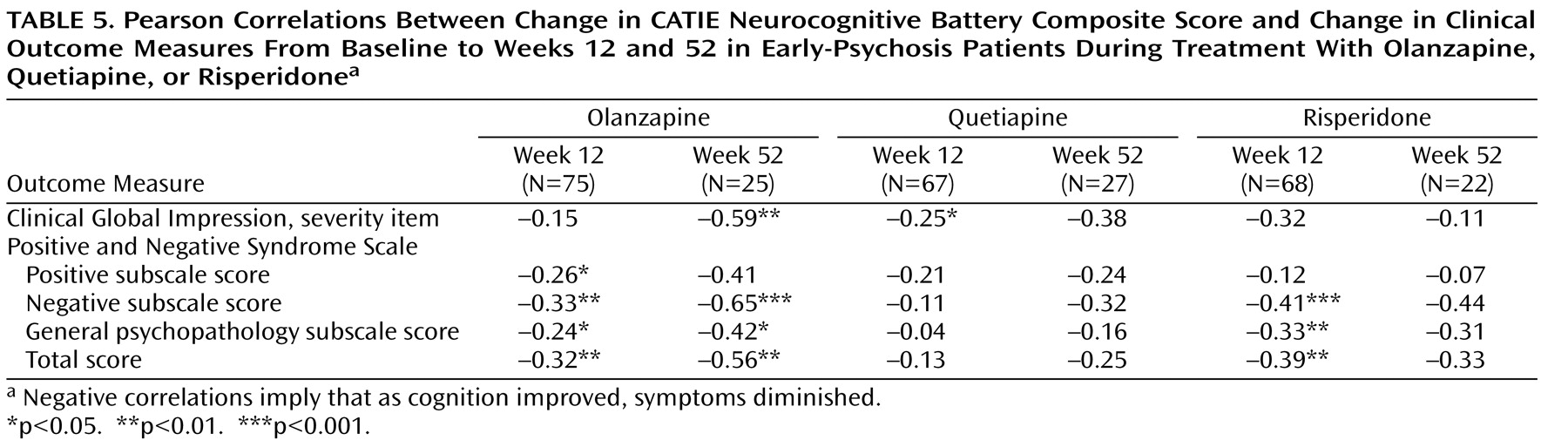
(9) . Grouped data from all studies of atypical antipsychotics suggest little additional cognitive benefit beyond the initial gains in the early phases (6–10 weeks) of treatment (unpublished 2004 analysis of R. Keefe and J. Cone). Our findings in this study support this view, as the magnitude of cognitive improvements across all domains was similar at 12 and 52 weeks, suggesting that most of the cognitive benefit of atypical antipsychotics occurs in the first few months of treatment. This finding may be particularly relevant for clinicians deciding whether to keep a patient on an antipsychotic treatment; it suggests that patients who do not demonstrate early cognitive benefit with a particular medication are unlikely to show benefit with continued treatment. After 12 weeks of treatment, all correlations between cognitive improvement and symptom improvement were less than 0.3, which is considered to be a medium effect, and most were closer to 0.1, which is a small effect
(43) . Thus, it is unlikely that the cognitive benefit of these antipsychotic treatments was caused by symptom improvement. The correlations with changes in symptoms were larger at 52 weeks, which suggests that patients who were able to continue treatment to the end of the study may have been more homogeneous and were improving across all symptom domains or that the clinical impact on cognition may require a longer period to fully manifest.
An important consequence of cognitive deficits in psychotic disorders is functional impairment
(11) . However, evidence that cognitive improvement with antipsychotic treatment leads to functional change is limited
(44) . In this study, patients with early psychosis who demonstrated cognitive improvement at 52 weeks also demonstrated functional benefit in social and occupational domains, which suggests a functional relevance for cognitive improvement. One caveat to this promising conclusion is that given the high dropout rate in this study, these data apply only to the patients who were able to stay in treatment and complete comprehensive assessments for 52 weeks, a group that comprised only 20% of the original sample. In addition, cognitive changes were not predictive of functional change when the analysis controlled for symptom change. Therefore, the functional benefits demonstrated in this study may be associated with cognitive and symptom improvement in patients who remain in treatment for substantial periods of time.
The correlations between cognitive change and change in side effect measures, such as tardive dyskinesia and extrapyramidal symptoms, including akathisia, were small and not statistically significant. Furthermore, patients who required anticholinergic medications or reported sleepiness did not differ from other patients in cognitive composite scores. These data suggest that with atypical antipsychotics, side effects are not an important determinant of cognitive functioning in relatively vulnerable early-psychosis patients with the doses used in this study.
One methodological issue that the data from this study address is the relative sensitivities of the CATIE neurocognitive battery, which was designed specifically for the CATIE project and requires about 90 minutes of testing time, and the BACS, a 35-minute assessment designed to be sensitive to treatment-related cognitive changes in clinical trials. In this study, the correlation between the composite scores for the two batteries was large at each assessment (r=0.84–0.90). The correlation between change in the BACS and change in the CATIE battery was also substantial: 0.70 at week 12 and 0.57 at week 52. The two batteries are similar in their sensitivity to deficits and antipsychotic treatment, association with changes in clinical and functional outcomes, and sensitivity to treatment-related side effects. However, the individual tests of the CATIE battery were slightly more sensitive to treatment differences. These results suggest that the BACS may save time and is equally sensitive to overall treatment differences, although the greater breadth of larger batteries, such as the CATIE battery, might be advantageous for elucidating more specific changes in individual cognitive domains and between treatments.