Individuals with schizophrenia have deficits in working memory
(1), defined as the ability to maintain and manipulate information over a short period of time
(2) . However, there is still controversy as to whether a deficit in central executive processing or domain-specific storage buffers underlies the deficit in working memory
(1,
3) . One of the most consistent findings in functional imaging studies of working memory in schizophrenia is the presence of abnormal activation in the dorsolateral prefrontal cortex
(4) . However, abnormal activation of the parietal cortex has also been observed in individuals with schizophrenia during working memory
(4), and a more detailed examination of parietal as well as prefrontal regions may help to resolve the controversy regarding the underlying basis of working memory deficits in schizophrenia.
An influential model put forth by Baddeley
(2) postulates that working memory has subcomponents that include 1) a short-term storage buffer for visual information, referred to as the visuospatial scratch pad, 2) a short-term storage buffer for verbal information, referred to as the phonological loop, and 3) a central executive component that guides the manipulation and transformation of information held within the storage buffers. The phonological loop can be further subdivided into at least two subcomponents: 1) articulatory rehearsal of phonologically based representations and 2) processing, coding, or storage of phonological representations. A number of studies suggest that articulatory rehearsal is particularly dependent on regions of the left ventrolateral prefrontal cortex, including Brodmann’s areas 44 and 45, but that the processing, coding, or storage of phonological representations is thought to depend on regions of the left posterior parietal cortex (e.g.,
5 –
8) .
There may also be at least two functionally dissociable regions of the left posterior parietal cortex that are responsive during working memory tasks
(7) . One of these regions, referred to as the left ventral inferior parietal cortex by Fiez, is thought to be a region that supports coding of phonological representations
(7) . This region is significantly more responsive in verbal than in nonverbal working memory tasks
(7,
9), is responsive in non-working-memory tasks that require phonological coding
(5,
10), and does not show increased activity as working memory load increases
(7) . According to Ravizza et al.
(7), this region has a z coordinate of between 10 and 30 mm, a y coordinate of –27 mm (15 mm), and an x coordinate of –52 (15 mm), according to the stereotaxic space atlas of Talairach and Tournoux
(11) . If individuals with schizophrenia were to have functional disturbances in the ventral inferior parietal cortex and/or ventrolateral prefrontal cortex, it would suggest impairments in language-related processes that may not be specific to working memory. A second, more dorsal region of the left posterior parietal cortex is also responsive in verbal working memory tasks. However, this region is also responsive to nonverbal working memory tasks, does not consistently show greater activity in response to words than to faces, increases activity as a function of working memory load
(7,
12), and is also responsive in tasks thought to require executive function more generally
(7,
13,
14) . According to Ravizza et al.
(7), the location of this region has a z coordinate between 32 and 52 mm, a y coordinate of –51 mm (15 mm), and an x coordinate of –34mm (±15 mm). If individuals with schizophrenia were to show functional activation deficits in the dorsal parietal cortex, it would suggest central executive-related disturbances that may impair both verbal and nonverbal working memory function. Such a result would be consistent with previous reports of impaired functioning in the dorsolateral prefrontal cortex (Brodmann’s area 46/9) in schizophrenia
(15) ; this is the region of the frontal cortex thought to support central executive functions of working memory
(16) .
The authors of a recent meta-analysis concluded that individuals with schizophrenia show consistent impairments across material types (e.g., faces, words) within working memory
(1), and a number of researchers have suggested that the primary dysfunction leading to working memory disturbances in schizophrenia arises from a defect of central executive function
(15,
17,
18) . However, other researchers have suggested that individuals with schizophrenia show a differential impairment in verbal working memory, possibly caused by a disturbance in the phonological loop
(19,
20) . Functional neuroimaging studies of working memory in individuals with schizophrenia consistently show abnormal activation in the dorsolateral prefrontal cortex
(4) . Given that many researchers believe that this brain region is critical for a number of executive functions, such findings are consistent with the hypothesis that working memory impairments in schizophrenia reflect, at least in part, deficits in central executive function. Further, few studies of working memory have shown impaired activation in the left ventrolateral prefrontal cortex, the region associated with articulatory rehearsal. However, many working memory studies in schizophrenia also show abnormal activation of the posterior parietal cortex
(4) .
To date, it has not been clear whether the parietal regions that show abnormal activation in schizophrenia correspond to the dorsal parietal cortex or the ventral inferior parietal cortex
(4) . This is in part due to the fact that the majority of working memory studies in schizophrenia have used only one material type, precluding the ability to identify parietal regions that are sensitive to material type. In three studies that did compare verbal and nonverbal working memory, it was not possible to clearly implicate either the dorsal parietal cortex or the ventral inferior parietal cortex. Walter and colleagues did not find any parietal regions that showed group differences in activation during working memory
(21) . We found bilateral parietal regions that showed impaired activation in both working and episodic memory tasks, but these regions were somewhat inferior to the canonical coordinates for the dorsal parietal cortex, and they were more posterior and medial than the canonical coordinates for the ventral inferior parietal cortex
(15) .
The goal of the current study was to provide further evidence regarding the pattern of activation in the frontal cortex (dorsolateral versus ventrolateral prefrontal cortex) and parietal cortex (dorsal versus ventral inferior parietal cortex) during working memory in schizophrenia. Using data from two prior studies of working memory, we sought to replicate previous findings of activation in the left ventrolateral prefrontal and ventral inferior parietal cortices in healthy individuals that was more responsive to verbal than nonverbal working memory, as well as dorsolateral prefrontal and dorsal parietal cortex activation that was equally responsive to both verbal and nonverbal working memory. Second, we examined whether individuals with schizophrenia showed material-sensitive activation in the left ventrolateral prefrontal and ventral inferior parietal cortices. Last, we examined whether individuals with schizophrenia showed abnormal prefrontal or parietal activation in response to both verbal and nonverbal working memory demands.
Method
Participants
The participants were 120 healthy comparison subjects and 57 individuals with DSM-IV schizophrenia from two prior studies
(15,
22) . The patients with schizophrenia were recruited from the St. Louis Metropolitan Psychiatric Center and its outpatient clinics. The healthy comparison subjects were recruited by using local advertisements in the same community as the individuals with schizophrenia. Potential participants were excluded for 1) meeting the DSM-IV criteria for substance abuse (severe) or dependence (any type) within the past 3 months, 2) having any clinically unstable or severe medical disorder, 3) having a history of head injury with documented neurological sequelae or resulting in loss of consciousness, 4) meeting the DSM-IV criteria for mental retardation, or 5) for comparison subjects, having a lifetime history of axis I psychiatric disorder or any first-degree relative with a psychotic disorder. Diagnostic information was collected by a master’s-level research assistant using the Structured Clinical Interview for DSM-IV
(23) and all available information from hospital records and corroborative family sources. Handedness was assessed with the Edinburgh Handedness Inventory
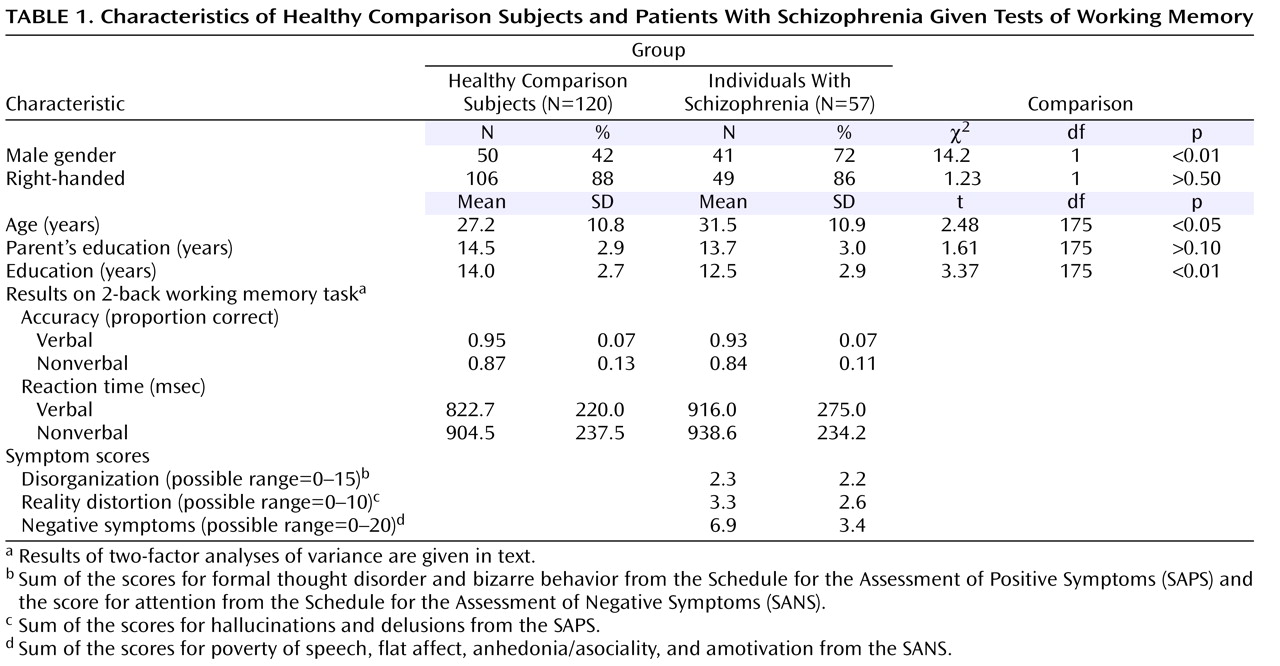
(24) . Demographic information is displayed in
Table 1, which shows that the comparison subjects and the individuals with schizophrenia did not differ in parental education or handedness. The comparison subjects were slightly younger than the patients with schizophrenia. However, findings from analyses conducted on a subgroup of 112 comparison subjects and 56 individuals with schizophrenia matched on age did not differ from the results with the full study group.
Tasks and Materials
Participants in both studies performed the same version of the 2-back task
(15,
22) . Stimuli appeared one at a time on the screen, and the participants were told to push one button any time they saw a stimulus that was the same as the stimulus they had seen two trials back, and a different button if not. The stimuli for the verbal tasks were visually presented words, 3–10 letters in length. The stimuli for the nonverbal tasks were unfamiliar faces that were hard for participants to verbally label. Each working memory run lasted 4.25 minutes and included four task blocks of 16 trials and three fixation blocks of 10 trials interleaved. The participants also completed verbal and nonverbal encoding tasks, which we used as conditions with low demands on working memory, against which to compare the 2-back conditions. In study 1, this was an intentional encoding task, in which the participants were told to remember a series of items for a later memory test. In study 2, this was an incidental task in which the participants made abstract/concrete judgments (words) or gender judgments (faces). The results of the comparisons of the 2-back task and encoding task were similar for studies 1 and 2. Thus, data from the two studies were combined. The order in which the participants performed the verbal versus nonverbal versions was counterbalanced across participants.
Scanning
Scanning was performed on the 1.5-T Siemens Vision at the Research Imaging Center of the Mallinckrodt Institute of Radiology at the Washington University School of Medicine. In both studies, the functional data were collected in runs using an asymmetric spin-echo echo-planar sequence sensitive to blood oxygenation level-dependent contrast (T 2 *) (TR=2500 msec, TE=50 msec, field of view=24 cm, flip angle=90°). During each functional run, 102 sets of oblique axial images were acquired parallel to the anterior-posterior commissure plane (3.75×3.75-mm in-plane resolution). In study 1, 16 8-mm-thick slices were acquired in each image. For study 2, 19 7-mm-thick slices were acquired in each image. Structural images for both studies were acquired by using a coronal MP-RAGE (magnetization prepared rapid gradient echo) three-dimensional T 1 -weighted sequence (TR=9.7 msec, TE=4 msec, flip angle=10°; voxel size=1×1×1.2 mm). These structural images were used for between-subject registration (described in the following) and anatomic localization.
A number of preprocessing steps were completed before statistical analyses: 1) compensation for slice-dependent time shifts, 2) elimination of odd/even slice intensity differences due to interpolated acquisition, 3) realignment of all data acquired for each participant within and across runs to compensate for rigid body motion
(25), 4) intensity normalization to a whole-brain mode value of 1000, and 5) spatial smoothing with an 8-mm full width at half maximum Gaussian kernel. The functional data were automatically transformed into the stereotaxic atlas space of Talairach and Tournoux
(11) by computing a sequence of affine transforms, and they were then resampled to 3-mm cubic voxels.
Statistical Analysis
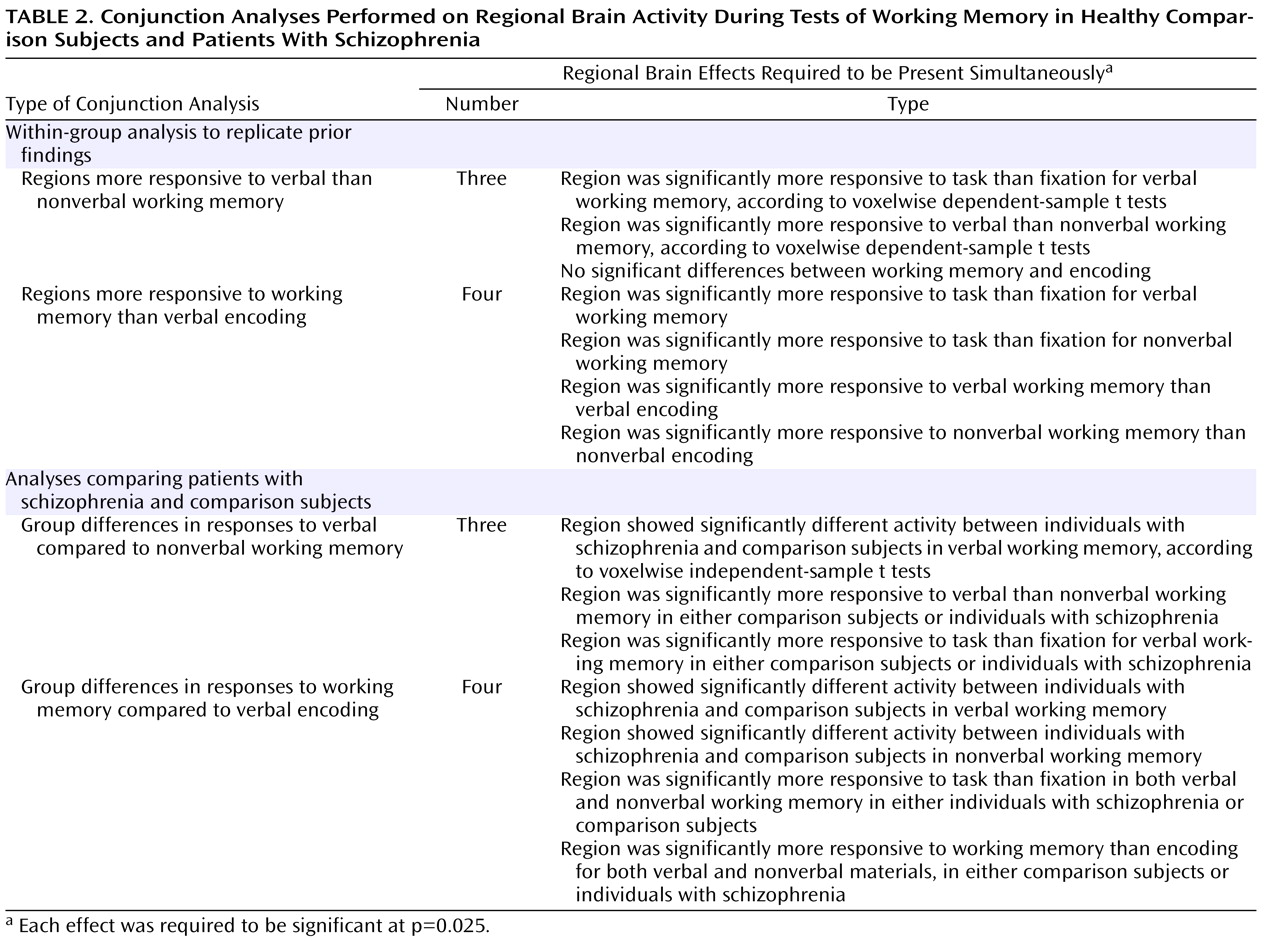
For each participant, we estimated the magnitude of task-related activation in each voxel by means of a general linear model using a box-car function convolved with a canonical hemodynamic response, with separate estimates for each material type (e.g., working memory task with words, working memory task with faces). These estimates were then entered into appropriately designed analyses of variance (ANOVAs) and t tests (to be described) that treated subjects as a random factor. To control the whole-brain false positive rate, we used a cluster-size threshold of 10 contiguous voxels and a per-voxel alpha of 0.000625 or less. The analyses presented in the next section were conjunction analyses, in which we required multiple effects to be significant simultaneously. Each effect was required to be significant at a p value of 0.025, resulting in a combined significance of 0.000625 (0.025×0.025) for each voxel when two conjunctions were required and a combined significance of 0.000016 (0.025×0.025×0.025) when three were required. Such procedures have been used previously on a number of occasions and are described in detail elsewhere
(26) .
Results
Behavioral Data
Analyses of the two groups combined produced the same results as found previously in each group separately
(15,
22) (see
Table 1 for characteristics). A two-factor ANOVA on accuracy with stimulus type (word, face) and group (comparison subjects, patients) as factors indicated a main effect of stimulus type (F=25.2, df=1, 175, p<0.001) and a main effect of group (F=40.6, df=1, 175, p<0.001) but not a significant interaction between group and stimulus type (F=0.8, df=1, 175, p>0.30). The two-factor ANOVA for reaction time indicated a nearly significant main effect of stimulus type (F=3.7, df=1, 175, p=0.06) but no significant main effect of group (F=1.8, df=1, 175, p>0.15) and no significant interaction between group and stimulus type (F=1.8, df=1, 175, p>0.15).
fMRI Data
Within-group analyses to replicate prior research
To identify regions that were more responsive to verbal than nonverbal processing demands, we used the conjunction of three effects, as shown in
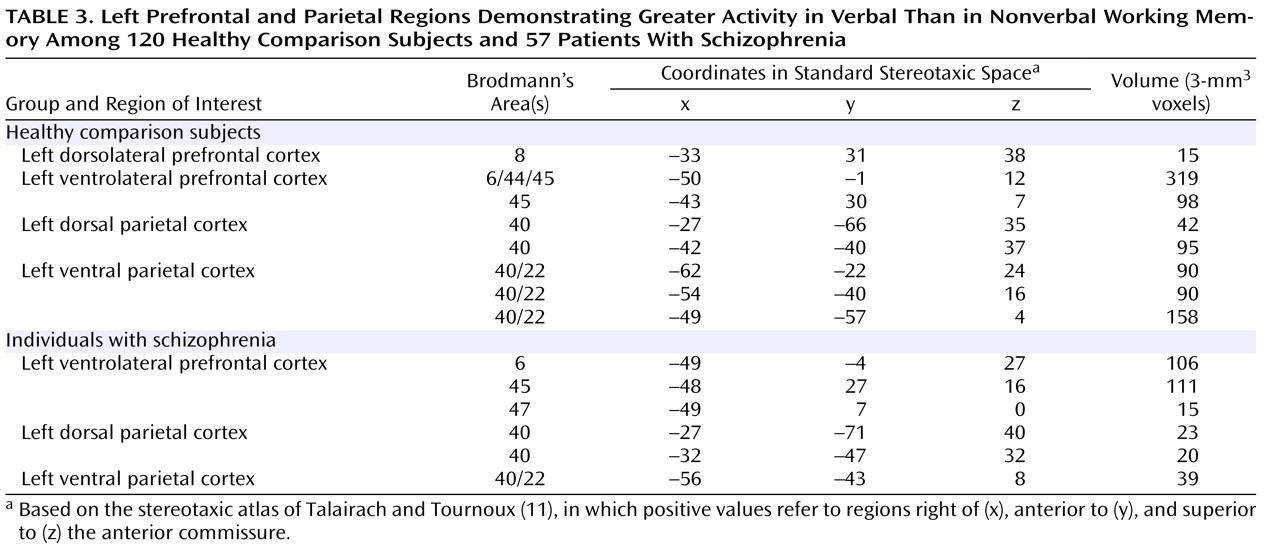
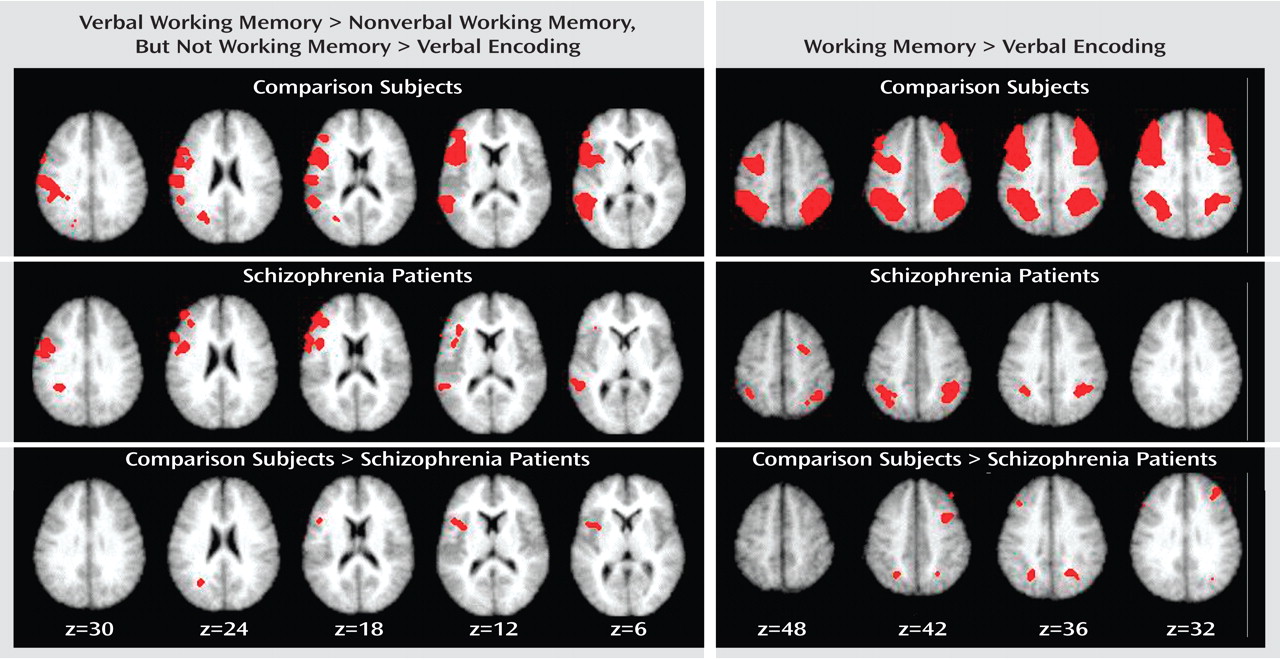
Table 2 . We first conducted these analyses for the comparison subjects alone. As shown in
Table 3 and
Figure 1, we found clear evidence for a number of prefrontal and parietal regions that were more active for verbal as compared to nonverbal working memory. Of the prefrontal regions, two were in the ventrolateral prefrontal cortex and one was in the superior frontal cortex. Of the parietal regions, two clearly correspond to the area of the left ventral inferior parietal cortex identified by Fiez. However, we also found additional, more dorsal parietal regions showing similar patterns of activity. We repeated these same analyses in the individuals with schizophrenia. As shown in
Table 3 and
Figure 1, the individuals with schizophrenia also showed a number of prefrontal regions that were more responsive to verbal than nonverbal working memory, all in the ventrolateral prefrontal cortex. In addition, the individuals with schizophrenia showed a number of parietal regions that were more responsive in verbal than nonverbal working memory, one in the ventral inferior parietal cortex as defined by Ravissa et al.
(7), one in the dorsal parietal cortex as defined by Ravissa et al.
(7), and one in a more posterior region of the dorsal parietal cortex.
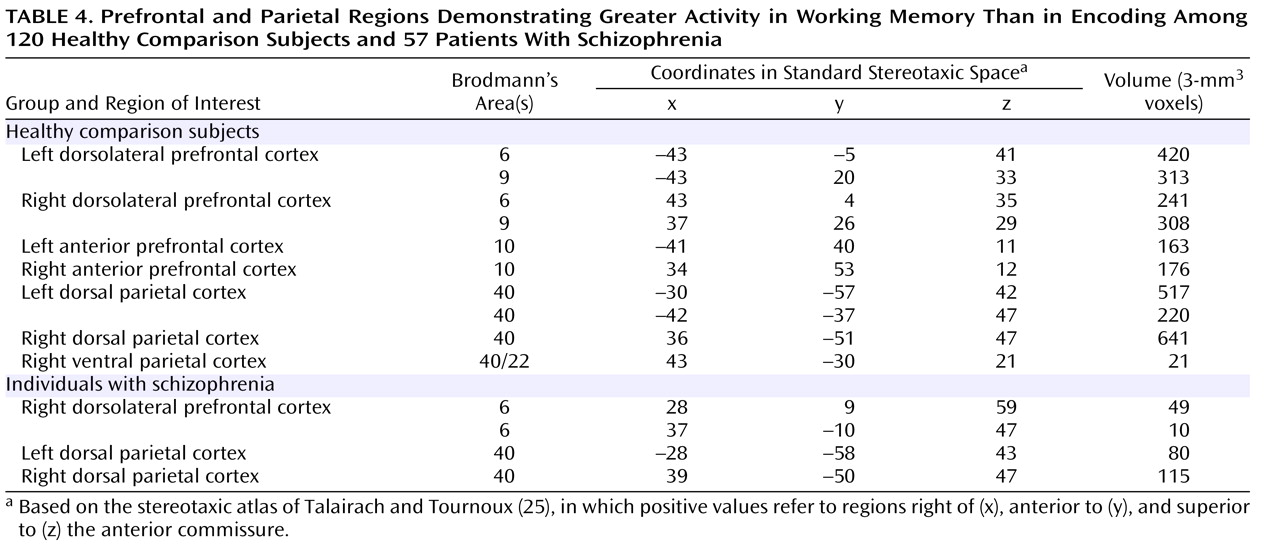
To identify regions that were responsive to working memory demand, we used the conjunction of four effects, as listed in
Table 2 . As shown in
Table 4 and the right side of
Figure 1, we found robust activity for this analysis in both the left and right dorsolateral prefrontal cortex and the dorsal parietal cortex among the comparison subjects, although we also found a small region of the right ventral inferior parietal cortex showing a similar pattern. We repeated these analyses on the individuals with schizophrenia and again found bilateral dorsal parietal cortex regions that showed significantly greater activity in both verbal and nonverbal working memory. We also found regions in the right prefrontal cortex that showed greater activity for working memory than encoding, but these were more superior than the ones activated in the comparison subjects and did not include Brodmann’s area 46 or 9.
Analyses directly comparing individuals with schizophrenia and comparison subjects
To look for group differences in the identified regions that were more responsive to verbal than to nonverbal working memory, we used the conjunction of three effects, as shown in
Table 2 . Given that the last two effects were identical to those used to identify regions in the ventrolateral prefrontal and ventral inferior parietal cortices within each group separately, this analysis essentially examined group differences in the regions presented in
Table 3 . This analysis revealed two regions in which activity differed significantly between the individuals with schizophrenia and the comparison subjects. One was a left dorsal parietal cortex region (Brodmann’s area 40, x=–24, y=–59, z=32), and one was a left ventrolateral prefrontal cortex region (Brodmann’s area 6, x=–43, y=10, z=12). Both of these regions showed a larger difference between words and faces in the comparison subjects than in the patients.
To look for group differences in the identified regions of the dorsolateral prefrontal and dorsal parietal cortices that were equally responsive to verbal and nonverbal working memory, we used the conjunction of four effects, as shown in
Table 2 . Again, given that the last two effects were identical to those used to identify regions within each group separately, this analysis examined group differences in the regions presented in
Table 4 . This analysis revealed five regions where activity differed significantly between the individuals with schizophrenia and the comparison subjects. Three were in the dorsolateral prefrontal cortex, one on the left (Brodmann’s area 9, x=–40, y=24, z=33) and two on the right (Brodmann’s area 8, x=36, y=32, z=40; Brodmann’s area 9, x=34, y=3, z=37). Two were in the dorsal parietal cortex, one on the left (Brodmann’s area 40, x=–24, y=–61, z=33) and one on the right (Brodmann’s area 40, x=23, y=–60, z=36). In all regions, the comparison subjects exhibited significantly greater task-related activity than the individuals with schizophrenia for both verbal and nonverbal working memory.
We next conducted analyses to examine the hypothesis that individuals with schizophrenia show abnormal connectivity between regions in the dorsolateral prefrontal and dorsal parietal cortices that are responsive to working memory demands. Among the comparison subjects, activity in the two dorsal parietal regions showing group differences in both word and face working memory was significantly correlated with activity in the dorsolateral prefrontal regions showing the same pattern of group differences (0.21<r<0.47). The individuals with schizophrenia also showed significant correlations between activity in these regions of the dorsal parietal and dorsolateral prefrontal cortices (0.28<r<0.45), except for a nonsignificant correlation between the left dorsal parietal region and one of the right dorsolateral prefrontal regions (r=0.21). The magnitudes of the correlations between the comparison subjects and individuals with schizophrenia did not differ significantly. However, the pattern differed somewhat between groups. In the comparison subjects, the right dorsal parietal regions were significantly more correlated with the right dorsolateral prefrontal region (r=0.43) than with the left dorsolateral prefrontal region (r=0.21). In addition, among the comparison subjects, the left dorsal parietal region was significantly more correlated with one of the right dorsolateral prefrontal regions (r=0.40) than with the left dorsolateral prefrontal region (r=0.22). Among the patients, the pattern of correlations did not differ significantly between the left and right dorsolateral prefrontal cortex. However, there was a tendency for both regions in the dorsal parietal cortex to have stronger correlations with the left dorsolateral prefrontal cortex (0.31<r<0.45) than with the right dorsolateral prefrontal cortex (0.21<r<0.38).
Work on the neural systems supporting working memory in schizophrenia suggests that the degree and pattern of abnormal brain activation may differ depending on the level of behavioral impairment in the patient group (e.g., 27). Thus, we identified subgroups of the comparison subjects (N=78) and individuals with schizophrenia (N=42) who were matched on accuracy and reaction time, and we reexamined activity in the regions demonstrating group differences. All regions that showed significant group differences in the full study group continued to show significant group differences, in the same direction, in the performance-matched group. However, the effect sizes for the two regions showing group differences in material-specific effects (effect sizes of 0.45 and 0.50) were slightly reduced in the performance-matched group (effect sizes, 0.41 and 0.43). In contrast, the effect sizes for four out of the five regions showing group differences in both word and face working memory were similar or increased in the performance-matched group (effect sizes of 0.47, 0.47, 0.47, 0.49 versus 0.47, 0.48, 0.56, 0.51). The remaining region changed from an effect size of 0.39 to 0.37.
Discussion
The results of the current study provide further evidence regarding the normal functions of subregions of the posterior parietal cortex and prefrontal cortex, as well as abnormalities in the function of these regions in schizophrenia. First, in terms of the normal function of the posterior parietal and prefrontal regions, our results replicate findings from previous studies. Specifically, we found evidence for the presence of at least two subregions of the prefrontal and parietal cortices that play different roles in working memory. In healthy subjects, although not individuals with schizophrenia, we found regions of the bilateral dorsolateral prefrontal cortex that showed greater activity in working memory than in encoding but did not show consistently greater activity in verbal than in nonverbal working memory
(28,
29) . In contrast, in both the comparison subjects and individuals with schizophrenia, we found regions of the ventrolateral prefrontal cortex that
did show significantly greater activity in verbal versus nonverbal working memory
(28,
29) . Like Ravizza et al.
(7), we found that the bilateral dorsal parietal cortex showed greater activity under a high versus low working memory demand for both the comparison subjects and individuals with schizophrenia, but it did not consistently show greater activity in verbal than nonverbal working memory. In contrast, for both the comparison subjects and individuals with schizophrenia, the left ventral inferior parietal cortex showed robustly stronger activity in verbal than in nonverbal working memory but did not show greater activity for working memory than for encoding conditions. These findings are consistent with the suggestion that the ventral inferior parietal cortex plays a role in phonological processing
(7), while the dorsal parietal cortex is associated with a frontal-parietal executive system
(7,
13,
14,
30,
31) .
Our results also suggest that the ventrolateral prefrontal cortex/ventral inferior parietal cortex and the dorsolateral prefrontal cortex/dorsal parietal cortex show differential impairments in individuals with schizophrenia. In schizophrenia patients, we found regions of the left ventrolateral prefrontal and ventral inferior parietal cortices that were more responsive to verbal than nonverbal working memory but not more responsive to working memory than to encoding. In addition, small bilateral regions of the dorsolateral prefrontal and dorsal parietal cortices were more responsive to working memory than to encoding, although not necessarily more responsive to verbal than nonverbal working memory. When we conducted direct group contrasts, we found strong evidence for group differences in the dorsolateral prefrontal cortex, but only one small region in the ventrolateral prefrontal cortex showed a group difference. Further, we found evidence for altered task-related activation only in the bilateral dorsal parietal cortex for both verbal and nonverbal working memory, and not in the left ventral inferior parietal cortex. Given hypotheses that the dorsolateral prefrontal and dorsal parietal cortices are involved in a frontal-parietal executive system, such results are consistent with the hypothesis that the primary deficit in working memory among individuals with schizophrenia involves a disturbance of central executive function
(1,
15,
32,
33) . Further, the findings that the patients with schizophrenia and healthy subjects showed the same pattern of activation in the ventrolateral prefrontal and ventral inferior parietal cortices and that the group differences in the ventrolateral prefrontal cortex were small compared to those in the dorsolateral prefrontal cortex suggest that schizophrenia does not involve a specific deficit in the verbal aspects of working memory
(3,
20,
34,
35) . Such results suggest that individuals with schizophrenia will show impairments on a wide range of real-life tasks that involve both verbal and nonverbal aspects of working memory, including occupations that may require verbal or nonverbal working memory or both. Further, such results suggest that any intervention techniques, either pharmacological or behavioral, should focus on amodal central executive components of working memory, rather than modality-specific components of working memory.
Finding impaired activation among individuals with schizophrenia in a region of the parietal cortex thought to be part of a frontal-parietal executive system is consistent with numerous prior reports of altered functional connectivity between frontal and parietal regions among individuals with schizophrenia (e.g.,
36,
37) . Notably, the regions of the parietal cortex showing altered functional connectivity with the frontal cortex in these prior studies were in the area of the dorsal parietal cortex described by Ravizza et al.
(7) and identified as impaired in schizophrenia here. Further, our correlational analyses suggest a link between the degree of impaired activation in dorsolateral prefrontal and dorsal parietal regions among the patients, consistent with the hypothesis that these regions are part of the same neural system. However, further work is needed to characterize the functional connectivity between the dorsolateral prefrontal and dorsal parietal cortices across the course of working memory performance in schizophrenia and to examine whether this may vary as a function of working memory load. Taken together, such findings raise interesting questions about the role of a disturbance in dorsal parietal cortex function and executive control processes during working memory tasks in schizophrenia. One hypothesis is that the dorsal parietal cortex makes a specific contribution to the temporal coding of items within working memory by using magnitude codes
(13) in coordination with the dorsolateral prefrontal cortex
(38) and that it is this coordinated activity of the dorsal parietal and dorsolateral prefrontal cortices that is disturbed in schizophrenia. Such a hypothesis is supported by evidence that individuals with schizophrenia have difficulties coding the temporal order of items within a working memory task
(22,
39) . However, further work will be needed to establish a tighter link between deficits in the temporal coding of order in working memory among individuals with schizophrenia and disturbances in the functional activation and connectivity of the dorsal parietal cortex.
There were several limitations to the current study. First, we did not have an explicit manipulation of working memory load, such as directly comparing 0-, 1-, and 2-back levels of the N-back task. However, our comparison of the 2-back working memory task to the encoding tasks still captured many of the same distinctions one would want to capture between different levels of the N-back task (i.e., updating, flushing the memory buffer, and the degree of active maintenance). It is true that our verbal encoding task likely engaged a significant amount of phonological processing, perhaps more so than a low-load working memory task (e.g., 0-back working memory). However, this may actually be seen as a positive feature, as it helps to further dissociate other aspects of working memory from phonological processing. A second limitation was that all of the individuals with schizophrenia were currently taking antipsychotic medications. However, a priori one would not necessarily predict that dorsal versus ventral inferior parietal cortex regions should be differentially sensitive to the influence of antipsychotic medication. A third potential limitation was that there was a higher proportion of men among the individuals with schizophrenia than among the comparison subjects. However, reanalysis of the data with gender as an additional factor did not change the results, and gender did not interact with group. Further, recent work from our laboratory has demonstrated that men and women show the same patterns of material-sensitive activation in both the frontal and parietal cortices, suggesting that sex differences should not alter patterns of material-sensitive activity
(40) .