The early course of schizophrenia is often dominated by florid psychotic symptoms such as hallucinations or delusions (positive symptoms), but persistent difficulties with emotional expression (flattened affect), social communication (alogia), and motivation (avolition) often become the most debilitating aspects of schizophrenia as time passes
(1–
3). These latter, so-called negative symptoms are often most prominent in patients with schizophrenia who have poorer premorbid adjustment, earlier onset, and greater illness chronicity
(4). Negative symptoms remain relatively unresponsive to most antipsychotic medications (perhaps even to atypical drugs such as clozapine)
(5). Furthermore, negative symptoms interfere with psychosocial rehabilitation efforts and often persist unchanged (if not worsening) throughout the course of the illness
(6).
The persistent, invariant quality of negative symptoms might lead one to predict that their etiopathogenesis might be easier to identify than positive symptoms, which often vary unpredictably in quality, duration, and amount. Because the negative symptoms in patients with schizophrenia are phenomenologically similar to the disruptions of affect, cognition, and motivation in neurological patients with frontal lobe lesions
(7,
8), it has been speculated that these symptoms arise from dysfunction of the frontal lobes, particularly the dorsolateral prefrontal cortex
(9–
11).
Previous attempts to correlate negative symptoms with indirect measures of cortical neuronal pathology—including structural imaging, neuropsychological tests of dorsolateral prefrontal cortex function, regional cerebral blood flow (rCBF), and evoked electrophysiological responses—have had some success (e.g., reference
12), but replication has been inconsistent. For example, frontal lobe structural measurements have been found to predict negative symptoms in some studies
(9,
13–
16), but not others
(17–
21).
Patients with schizophrenia show greater impairments on tests of cognition thought to rely on intact dorsolateral prefrontal cortex function, such as the Wisconsin Card Sorting Test
(22–
24) and working memory tasks
(25), and these deficits have been associated with negative symptoms
(26–
30). Moreover, in some reports, reduced dorsolateral prefrontal cortex blood flow at rest also predicted greater negative symptoms
(31�35). Neurophysiological measures of dorsolateral prefrontal cortex function have also been reported as abnormal in schizophrenia, including abnormal eye tracking
(36), abnormal EEG patterns
(37), and disruption of the normal coherence between the dorsolateral prefrontal cortex and other brain regions
(38). A sizable functional neuroimaging literature, although at times contradictory
(39), has largely reported reduced physiological capacity of the dorsolateral prefrontal cortex in response to cognitive challenge (e.g., references
40–
44).
None of these indirect measures, however, speaks to the integrity of prefrontal neurons per se; the different findings might also reflect activity in prefrontal inputs. For example, Sabri et al.
(35) found that although negative symptoms correlated with bilateral frontal rCBF, negative symptoms also correlated with bilateral temporal, cingulate, left parietal, basal ganglia, and thalamic rCBF at rest.
In contrast to the modest in vivo evidence for a relationship between negative symptoms and frontal lobe dysfunction, evidence of dorsolateral prefrontal cortex neuronal pathology is compelling. Postmortem neuropathological human studies have found prefrontal gray matter abnormalities, including reduced neuropil volume with increased neuronal density in the dorsolateral prefrontal cortex
(45), decreased inhibitory input from prefrontal chandelier cells onto dorsolateral prefrontal cortex pyramidal neurons
(46), and decreased abundance and activity of interneurons
(47,
48). In both medicated and untreated patients with schizophrenia, studies using proton magnetic resonance spectroscopy (
1H-MRS and
1H-MRSI) have repeatedly found evidence of dorsolateral prefrontal cortex neuronal pathology reflected in reduced concentrations of the intraneuronal chemical
N-acetylaspartate (NAA)
(49).
Based on 1H-MRSI and postmortem evidence of dorsolateral prefrontal cortex neuronal pathology in schizophrenia, we hypothesized that if negative symptoms arise from such pathology, NAA measures should correlate with negative symptom ratings. In particular, larger reductions in dorsolateral prefrontal cortex NAA, and by inference greater neuronal pathology, should predict more severe negative symptoms. Since it is possible with 1H-MRSI to obtain NAA from many regions of brain, one would not predict similar regional correlations between NAA and negative symptoms if the cellular basis of the symptoms were regionally selective.
Method
We studied 36 patients with schizophrenia diagnosed according to DSM-IV criteria. There were six women (five were right-handed) and 30 men (26 were right-handed) with a mean age of 34 years (SD=8). Patients were recruited from the inpatient wards of the National Institute of Mental Health (NIMH) at St. Elizabeths Neuropsychiatric Research Hospital, Washington, D.C., and the Warren Magnuson Clinical Center, Bethesda, Md. In addition, patients were studied as part of the NIMH Clinical Brain Disorders Branch Sibling Study of Schizophrenia, Bethesda, Md.
The majority of patients (30 of the 36) were receiving a stable dose of antipsychotic medication at the time of scanning. The six medication-free subjects had been off all medicines for a minimum of 14 days. The remaining, medicated patients were evenly divided between typical (N=15) and atypical (N=15) antipsychotic treatment, with no acute change in dose for at least 14 days before scanning. The patients studied were chosen from a larger group of patients with schizophrenia on the basis of the presence of dorsolateral prefrontal cortex regional NAA measures that were of high quality as well as ratings that were obtained immediately after scanning.
Before participating, all subjects underwent a diagnostic interview with the Structured Clinical Interview for DSM-IV
(50). In addition, patients completed a screening questionnaire regarding past substance abuse and neurological illnesses followed by a full neurological examination and a structural magnetic resonance imaging (MRI) clinical examination. Exclusion criteria included history of active or recent (less than 6 months) substance abuse, history of clinically significant neurological illness, or abnormalities on MRI examination. After discussion of the risks and benefits of the MRI procedure and questioning to determine assent and understanding of the MRI procedure, all subjects gave written informed consent. The MRI protocol (91-M-0124) was approved by the institutional review board of the NIMH Intramural Program.
For a comparison group, we used our database of healthy subjects of similar age and sex. The comparison subjects were 28 women (27 were right-handed) and 45 men (34 were right-handed) men with a mean age of 32.2 (SD=8.1). The same inclusion and exclusion criteria were applied to all healthy comparison subjects.
Metabolite spectra from multiple brain regions were acquired by using
1H-MRSI as previously described
(51). A 1.5-tesla GE Signa scanner (GE Medical Systems, Milwaukee) was used. The technique acquires four 15-mm-thick oblique axial slices (TR=2200 msec, TE=272 msec, voxel size=7.5 × 7.5 × 15 mm). All subjects included in the analysis were free from substantial movement artifact. Spectra were analyzed by using in-house software (A. Barnett) and then converted to ratios for further analysis. Regions of interest were then drawn by using T
1-weighted scans acquired immediately after the
1H-MRSI acquisition with the group identity blinded as described previously
(49). Region of interest analysis was used to quantify NAA, creatine (creatine plus phosphocreatine), and choline-containing compounds.
In addition to the dorsolateral prefrontal cortex, metabolite ratios were obtained from the orbitofrontal cortex, hippocampal area (inclusive of the amygdala), thalamus, putamen, anterior and posterior cingulate, superior temporal gyrus, prefrontal white matter, occipital cortex, and centrum semiovale for both patients and healthy comparison subjects. Due to the oblique angle of acquisition chosen to maximize coverage of the hippocampal area, the parietal cortex was not imaged. Furthermore, where metabolite ratios could not reliably be determined, they were not entered into the statistical analyses (thus, the numbers are reported separately for all correlations in addition to the p values).
Ratios of NAA to creatine, NAA to choline-containing compounds, and choline-containing compounds to creatine were used for the correlation analysis. To determine if there were regional abnormalities in these ratios, the patients were compared with the normal subjects (N=73) by using a three-way repeated measures analysis of variance (ANOVA) with region of interest and hemisphere as the repeated measures followed by post hoc comparisons.
Following the MRI scanning session, subjects were rated with the Scale for the Assessment of Negative Symptoms (SANS)
(52) and the Psychiatric Symptom Assessment Scale
(53) by a psychiatrist (J.H.C.) who was blind to the
1H-MRSI data. Because the ANOVA did not identify a significant interaction of diagnosis by region of interest by side, product moment correlations (r) were computed between mean right and left hemisphere NAA-creatine ratio, NAA-choline ratio, and choline-creatine ratio in each region of interest and summary scores for the total SANS and the positive and negative subscales of the Psychiatric Symptom Assessment Scale. On the basis of our previous hypotheses of selective reduction in the dorsolateral prefrontal cortex
(49), all analyses in other regions were corrected by the method of Bonferroni to avoid type I error (corrected p=0.0005 [0.05÷99] [three metabolite ratios × 11 regions of interest × three rating scales]). Within the text, statistical significance for regions outside of the dorsolateral prefrontal cortex will be reported as a corrected p.
Results
The patients with schizophrenia had significant bilateral reductions in NAA/creatine (main effect for diagnosis: F=5.7, df=1, 98, p=0.02; diagnosis by region: F=2.3, df=10, 908, p=0.01; diagnosis by region by hemisphere: F=0.6, df=10, 908, p=0.80). Overall, post hoc comparisons revealed significant reductions only in the dorsolateral prefrontal cortex (F=4.7, df=1, 132, p=0.03) and hippocampal area (F=13.6, df=1, 126, p<0.001), as found previously
(54–
57). There were no other statistically significant regional differences found. On average, the patients were moderately affected in terms of negative symptoms (SANS mean score=17.6, SD=13.7, and Psychiatric Symptom Assessment Scale negative subscale mean score=4.5, SD=3.1), and overall pathology (Psychiatric Symptom Assessment Scale total mean score=22.2, SD=11.1). These two rating instruments were significantly correlated with each other (r=0.70, N=36, p≤0.001). On the remaining items of the Psychiatric Symptom Assessment Scale (i.e., positive symptoms), the patients were mildly to moderately ill (mean=16.2, SD=9.2).
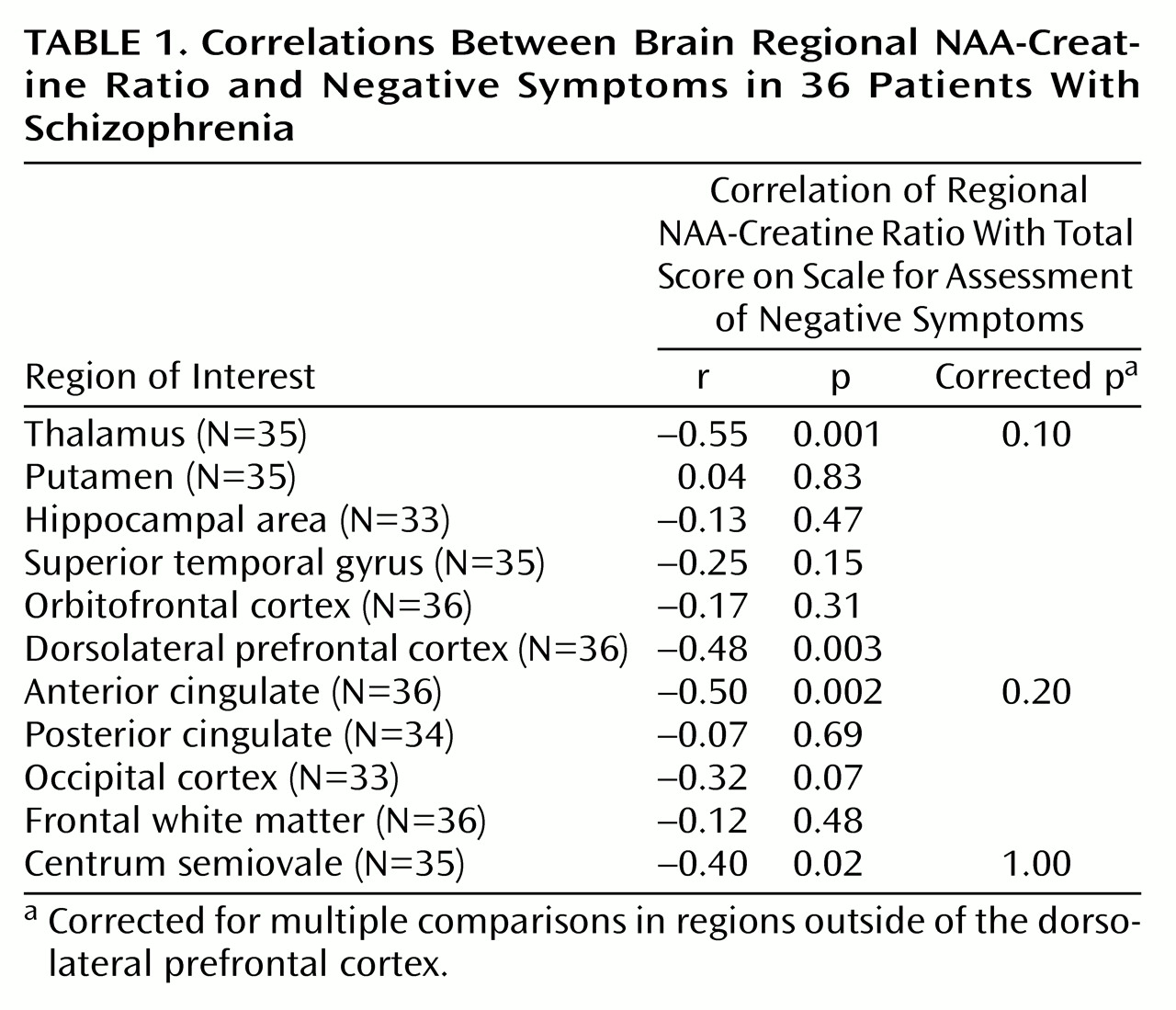
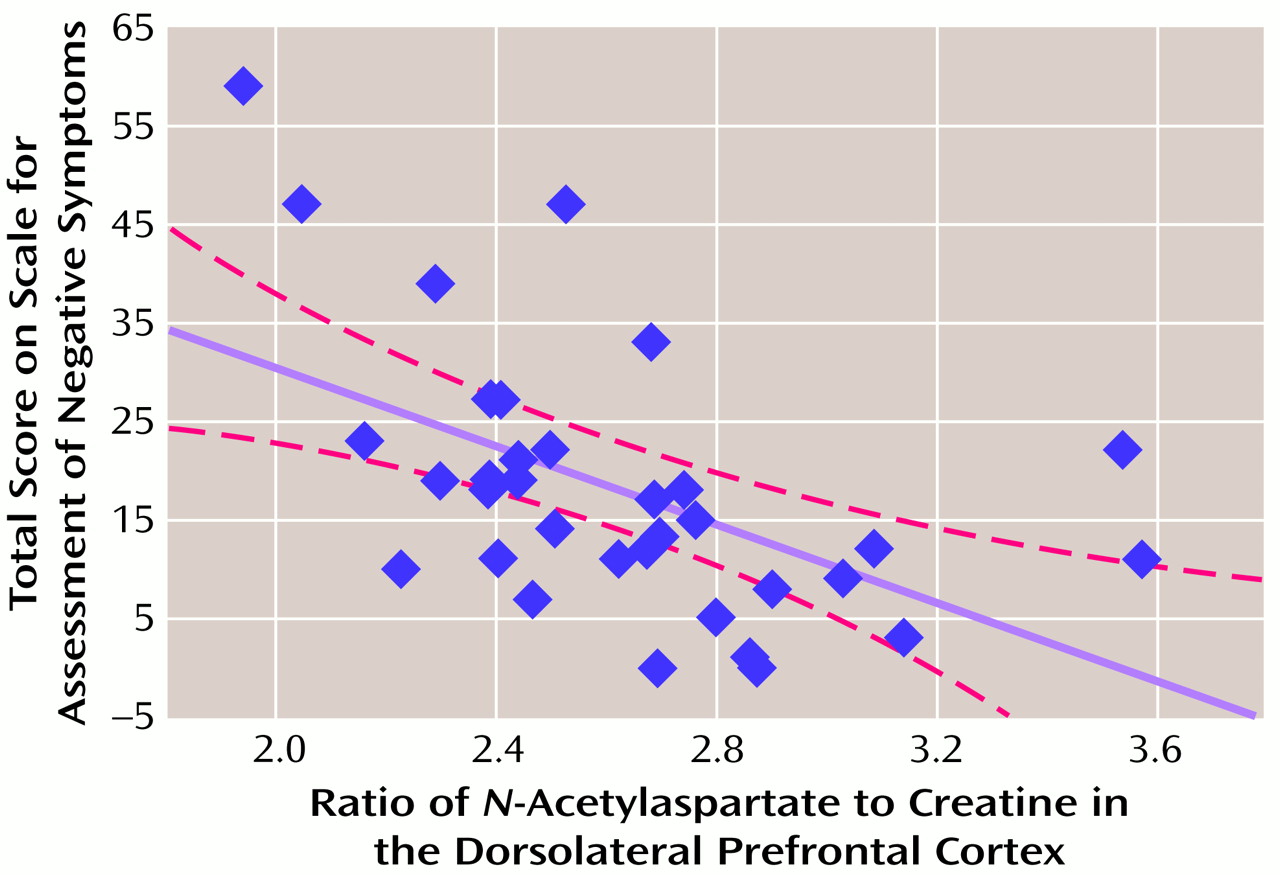
There was a significant relationship between dorsolateral prefrontal cortex NAA measures and negative symptoms. A negative correlation was found between dorsolateral prefrontal cortex NAA/creatine and SANS total scores (
Table 1 and
Figure 1) and the negative symptoms subscale of the Psychiatric Symptom Assessment Scale (r=–0.43, N=36, p=0.01). Neither dorsolateral prefrontal cortex NAA/choline nor the dorsolateral prefrontal cortex choline/creatine correlated with negative symptom measures.
Measures of positive symptoms did not correlate with any regional measure of NAA/creatine, NAA/choline, or choline/creatine. In spite of a between-group difference in NAA/creatine in the hippocampal area, there were no significant relationships between hippocampal area NAA/creatine and negative symptoms. However, there were negative correlations between SANS score and NAA/creatine in regions outside of the dorsolateral prefrontal cortex that share dense interconnectivity with the dorsolateral prefrontal cortex, including the thalamus and anterior cingulate (
Table 1). Although these regional correlations did not survive statistical correction for multiple comparisons, future studies aimed specifically at these regions should be pursued.
Discussion
We found a regionally selective relationship between evidence of dorsolateral prefrontal cortex neuronal pathology (as reflected in NAA measures) and negative symptoms in patients with schizophrenia. No regional measures predicted positive symptoms in this group of patients. These patients showed significantly lower NAA in only two regions—the dorsolateral prefrontal cortex and hippocampal region—when compared with normal subjects. Although hippocampal NAA measures were not found to predict negative symptoms, we did find evidence of involvement of a larger network in the production of negative symptoms. Specifically, the thalamus and anterior cingulate were implicated by negative correlations between NAA/creatine and negative symptom ratings (
Table 1).
Because these regions did not differ significantly between patients and healthy comparison subjects in this study, we cannot rule out the possibility that these represent spurious or secondary correlations. Nonetheless, their relationship to negative symptoms may arise from their dense interconnectivity with the dorsolateral prefrontal cortex. As a post hoc test of this supposition, we performed a forward stepwise multiple regression that included the dorsolateral prefrontal cortex, thalamus, and anterior cingulate NAA/creatine. Partial correlations revealed that neither the thalamus (partial correlation=–0.30, p=0.10) nor the anterior cingulate (partial correlation=–0.21, p=0.20) predicted negative symptoms (according to SANS scores) once the relationship between dorsolateral prefrontal cortex NAA/creatine and SANS scores was factored out. Nonetheless, these data suggest that multiple regions interact to produce negative symptoms.
The shared variance between dorsolateral prefrontal cortex pathology and negative symptoms was not large—approximately 23%. A number of factors may have contributed to this modest predictive value. Both
1H-MRSI and psychiatric rating scales are inherently imprecise measures
(49,
52). In addition,
1H-MRSI has a relatively low spatial resolution (about 1.4 cc/voxel with our method). Finally, although NAA is found almost exclusively within neurons
(58), the exact functional implications of reduced NAA levels remain unclear. NAA is found in highest concentration in pyramidal neurons in the dorsolateral prefrontal cortex
(59). In addition to serving as a storage form of aspartate, NAA is in the metabolic pathway for glutamate and, although not thought to be a neurotransmitter per se
(60), is capable of inducing calcium influx by means of
N-methyl-
d-aspartic acid receptors in vitro
(61). Thus, the measurement of NAA concentration in vivo is likely to reflect a number of potentially variable factors.
NAA reductions are clearly linked to a number of neurological disorders involving neuronal pathology
(60) and may be reversed by treatment in some instances
(62–
64). Thus, schizophrenia and its negative symptoms are not likely caused by NAA reductions per se, but, rather, NAA reductions are a marker for some other dorsolateral prefrontal cortex neuronal abnormality. NAA reductions are also not likely the result of a gross loss of neurons in the dorsolateral prefrontal cortex, given the wealth of postmortem human data suggesting that neuronal dropout is not characteristic
(65). To the extent that NAA concentrations likely correlate with the overall volume of neuronal soma and processes, the findings of reduced NAA measures are consistent with postmortem evidence of decreased neuropil and soma size
(45,
66).
Reduced NAA measures in the dorsolateral prefrontal cortex seem to predict a variety of phenomena associated with manifest illness. In addition to the present findings, dorsolateral prefrontal cortex NAA measures have been found to predict activation within the distributed neural network subserving working memory for patients with schizophrenia
(67), to predict baseline and amphetamine-induced striatal dopamine levels
(68,
69), and to predict the loss of dorsolateral prefrontal cortex neuronal efficiency during a parametric working memory task
(70). This particular predictive power of dorsolateral prefrontal cortex NAA measures is both striking and puzzling. However, given the widespread connectivity of dorsolateral prefrontal cortex neurons throughout the brain, these data may collectively suggest that certain dorsolateral prefrontal cortex neurons represent a population of effector neurons that act within larger cortical networks to determine many of the manifest state variables of schizophrenia.
In our patients, correlations between dorsolateral prefrontal cortex NAA measures and those in the thalamus and anterior cingulate support the supposition of a larger network, but this finding remains to be tested in future data samples. If greater dorsolateral prefrontal cortex neuronal pathology (lower NAA) can be used to predict greater negative symptoms, then these data not only will lend credence to the supposition that negative symptoms arise from dorsolateral prefrontal cortex neuronal pathology but also may provide an additional clinical tool for the assessment of neuropsychiatric patients.