The etiology of sexual orientation has been a focus of both scientific and general social interest. Twin studies
(1–
4) have suggested that familial factors, which may be genetic, have a substantial impact on sexual orientation. Three studies
(5–
7), with conflicting results, have examined whether a locus on the X chromosome influences sexual orientation.
The interpretation of prior twin studies of sexual orientation can be questioned on methodological grounds. Kallmann’s early study
(1) largely sampled subjects from correctional and psychiatric institutions. The two studies of Bailey and colleagues on male
(2) and female
(3) sexual orientation obtained subjects through advertisements in homophilic publications, raising concerns about representativeness. To our knowledge, only one study of sexual orientation has used a general population volunteer twin registry
(4). We here report results on sexual orientation in twin and nontwin sibling pairs ascertained as part of a U.S. national probability sample.
Method
Sampling
The data come from the MacArthur Foundation Midlife Development in the United States survey
(8,
9). This is a national telephone/mail survey carried out in 1995–1996 under the auspices of the John D. and Catherine T. MacArthur Foundation Network on Successful Midlife Development. A sample of 3,032 respondents, ranging in age from 25 to 74 years, was recruited from a random-digit-dial sampling frame of the coterminous United States. Only one respondent was selected from each eligible household. The survey was carried out in two phases: a telephone interview followed by a self-administered mail questionnaire. The phase 1 response rate was 70.0%, and the conditional phase 2 response rate was 86.8%, with an overall response rate of 60.8%. The entire protocol, including the obtaining of informed consent through oral assent before initiation of the telephone interview, was reviewed and approved by the Human Subjects Committee of Harvard University Medical School.
Twin pairs were recruited by using a separate two-part sampling design, the first part of which involved screening a representative national sample of approximately 50,000 households for the presence of a twin. This was done as part of ongoing national omnibus surveys conducted by ICR/AUS Consultants and Bruskin Associates. Respondents who indicated the presence of twins in the household or being part of a twin pair themselves were asked permission to be contacted by our research team for inclusion in the first national study of twins. The presence of a twin in the family was reported by 14.8% of the respondents, of whom 60.0% gave permission to be contacted for the twin study.
The second part of the twin sample design involved student recruiters from the University of Michigan contacting the twin households in order to recruit twins to participate in the survey. The cooperating twins were asked to provide contact information for their co-twins, who were also recruited by the students. The final response rate for the twin pairs varied according to whether the first contact was with a relative of the twin (20.6% response rate) or the twin him- or herself (60.4% response rate). The final twin sample included a total of 1,588 twins, resulting in 794 pairs, 763 of which came from distinct families. Fourteen families contributed two twin pairs, while one family contributed three twin pairs. Of these 794 pairs, 756 had a known zygosity and information on sexual orientation from both members.
Nontwin siblings were enrolled by sending a postcard to all respondents to the MacArthur Foundation Midlife Development in the United States survey, telling them of our interest in including siblings in the survey. The card asked them to provide contact information for their siblings and to communicate with their siblings about participation before the time when a recruiter would attempt contact. Since the family study was a secondary aim of the project, aggressive follow-up procedures were not employed. While the number of eligible respondents who provided us with the names and addresses of their siblings was low (19.7%), the cooperation rate for the sibling sample was much higher (69.3%). Of the 1,372 siblings referred to us from the survey participants, 951 were interviewed. The number of referred siblings recruited from any single family ranged from one to six, including one sibling from 272 families, two from 146 families, three from 75 families, four from 22 families, five from 10 families, and six from four families. Thus, these 951 siblings came from 529 families of original participants in the MacArthur Foundation Midlife Development in the United States survey, for a total sample of 1,480. However, of these newly recruited siblings, 81 did not complete the self-report questionnaire. Removing them from the sample left 19 individuals with no sibling, who were also excluded from the data analysis, so the final sample of nontwin siblings included 1,380 individuals.
Subject Characteristics
The twin and nontwin sibling subjects ranged in age from 25 to 74 years, with a mean of 47.2 (SD=12.6). The majority of the subjects were white (95.1%), and they were diverse with respect to educational attainment: 33.5% had graduated from college, 29.6% had some college education, 27.7% had graduated from high school, and the remaining 9.3% had less than a high school education. Among the nontwin sibling pairs, the mean age difference was 5.8 years (SD=4.5).
Assessment of Sexual Orientation
Sexual orientation was assessed by a single item in part 2 of the self-report questionnaire that read, “How would you describe your sexual orientation? Would you say you are heterosexual (sexually attracted only to the opposite sex), homosexual (sexually attracted only to your own sex), or bisexual (sexually attracted to both men and women)?” The response options were 1) heterosexual, 2) homosexual, and 3) bisexual. Because of limited statistical power, for the purpose of our present analyses we combined responses 2 and 3 to create a dichotomous rating of sexual orientation: heterosexual and nonheterosexual.
Zygosity Determination
Using as a test sample 230 pairs of unselected genotyped adult same-sex twins from the Virginia Twin Registry, we examined the common set of eight standard zygosity self-report items that were identical or highly similar across the two data sets. We obtained a linear discriminant function from the “test” sample of our 230 genotyped pairs and then applied it to the same-sex twin pairs in the sample from the MacArthur Foundation Midlife Development in the United States survey. Good separation was obtained, as 86% of the pairs were assigned a probability of monozygosity of less than 10% or more than 90%. We left as unassigned the 3.5% of pairs with a probability of monozygosity of between 40% and 59%, and these pairs were excluded from further data analysis.
Statistical Analysis
We assessed twin (and sibling) resemblance in three ways. Proband-wise concordance was defined as the proportion of co-twins of nonheterosexual twins who were themselves nonheterosexual. We also calculated the odds ratio, which is the ratio of the odds of being nonheterosexual among co-twins of nonheterosexual twins to the odds of being nonheterosexual among co-twins of heterosexual twins.
We used a liability-threshold model to estimate the genetic and environmental contributions to sexual orientation. For categorical characteristics, such as sexual orientation, the estimates are for the resemblance of twins in a pair for their liability to develop a nonheterosexual versus heterosexual sexual orientation
(10). Liability is assumed to be continuous and normally distributed in the population, with individuals who exceed a theoretical threshold expressing a nonheterosexual sexual orientation. We also present the tetrachoric correlation, defined as the correlation in members of twin or sibling pairs for the liability to nonheterosexual versus heterosexual sexual orientation
(10,
11).
We initially assumed four sources of individual differences in liability in our modeling: additive genes (abbreviated “A”), common or familial environment that affects the similarity of all twin/sibling pairs (C), a special twin environment that influences the similarity of twins only (T), and individual-specific environment (E). However, in all our models, estimates of T were zero because, contrary to expectation, sibling resemblance for sexual orientation was modestly greater than that seen in the dizygotic twins. Therefore, where necessary, we combined dizygotic twins and siblings into a single category and estimated only A, C, and E. Models were fit with maximum likelihood estimation by using the Mx structural modeling program
(12). Because of our low power for discriminating between models, we chose to present parameter estimates from only the full (ACE) model and divided, in our analyses, multiple sibships into all possible sibling pairs. This approach can be expected to obtain accurate parameter estimates but may underestimate sampling errors
(13).
The equal-environment assumption requires that members of monozygotic and dizygotic twin pairs be equally similar in their exposure to environmental factors of etiologic relevance to the trait being studied. If the environments of dizygotic twins were less similar than those of monozygotic twins, this would lead to an overestimation of genetic influence. We examined this possibility in two ways: by examining standard measures of the environmental similarity of twins in childhood
(14) and by examining the frequency of current contact between the members of the twin pair in adulthood. Using logistic regression and controlling for zygosity, we examined whether the mean level of childhood or adult environmental similarity reported by the twin pair could predict the concordance status of the twin pair for sexual orientation.
Because of the rarity of nonheterosexual sexual orientation in this sample, we had no statistical power to examine gender differences in sexual orientation. Whether similar or distinct familial factors influence sexual orientation in males and females remains uncertain
(4,
15). Therefore, we conducted analyses with and without opposite-sex dizygotic twins and nontwin siblings. Furthermore, we performed analyses by using the traditional twin design (monozygotic and dizygotic twins) as well as maximizing our power by including together dizygotic twins and nontwin siblings (who both share, on average, 50% of their genes identical by descent).
Results
Among the total sample of 2,968 individuals, data on sexual orientation were missing for 61 (2.1%). Of the remaining 2,907, 81 (2.8%) reported a nonheterosexual sexual orientation. The rate was nonsignificantly higher in men (3.1%) than in women (2.5%) (χ2=1.10, df=1, p=0.30). With controls for age and gender, the rate of nonheterosexual sexual orientation did not significantly differ between the twins and nontwin siblings (χ2=0.18, df=1, p=0.92), between the monozygotic and dizygotic twins (χ2=0.04, df=1, p=0.93), or between the same-sex and opposite-sex dizygotic twins (χ2=0.44, df=1, p=0.51). When we controlled for zygosity, twin resemblance for sexual orientation was not significantly predicted by measures of the similarity of their childhoods (χ2=1.02, df=1, p=0.31) or adult environments (χ2=2.47, df=1, p=0.12).
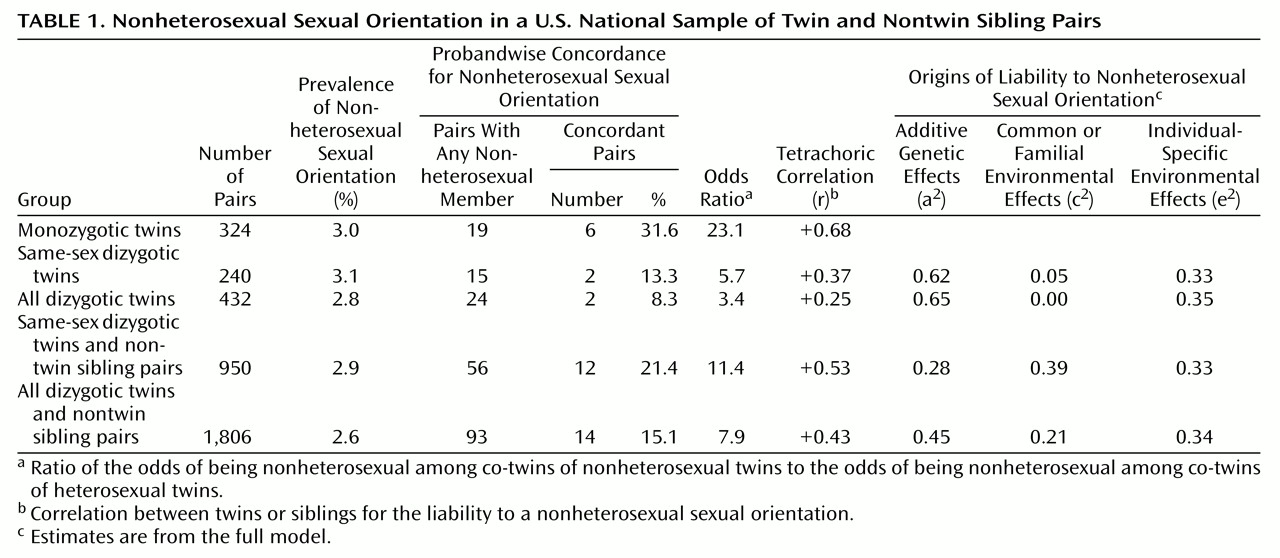
As assessed by proband-wise concordance, odds ratios, or tetrachoric correlations, pair resemblance for sexual orientation was substantially greater in the monozygotic twins than in any of the groups of dizygotic twins and nontwin siblings (
Table 1). Both in the dizygotic twin pairs alone and in the dizygotic twins plus nontwin sibling pairs, the resemblance for sexual orientation was greater in the same-sex pairs than in the opposite-sex pairs.
We performed biometrical twin modeling with the monozygotic twins and each of the four possible comparison groups of dizygotic twins and nontwin sibling pairs. Using the full model, we found estimates of the heritability of liability of sexual orientation ranging from 0.28 to 0.65. The estimates of the impact of familial environment were lower and ranged from 0.00 to 0.39.
Discussion
As in previous studies (e.g., references
15,
16), sexual orientation, as assessed by self-report questionnaire in a U.S. national sample of twin and sibling pairs, demonstrated substantial familial aggregation. In accord with findings from prior twin studies
(1–
4), resemblance for sexual orientation was greater in monozygotic twins than in dizygotic twins or nontwin sibling pairs. These results suggest that genetic factors may provide an important influence on sexual orientation. The concordance rate for nonheterosexual sexual orientation in monozygotic twins found in this sample (31.6%) is similar to that found in the one previous general population twin study
(4) but lower than the approximately 50% concordance rates found in the two previous studies that ascertained twin pairs through homophilic publications
(2,
3). These results suggest that twin pairs concordant for sexual orientation may be more likely to respond to such ads than are twin pairs discordant for sexual orientation.
These results should be interpreted in the context of important methodologic strengths and weaknesses of this sample. Three strengths are noteworthy. First, in contrast to prior twin studies of sexual orientation, this study was based on a national probability sample. One previous study used a general population sample
(4), but that was derived from a volunteer twin registry. Second, we assessed sexual orientation by questionnaire, which, with its relative anonymity, may be more likely than personal interview to elicit accurate responses on something as sensitive as sexual orientation
(17–
19). In their large survey of sexual practices in the United States, Laumann et al.
(20) included an item similar to the one used in our survey as part of their self-report questionnaire. They reported rates of nonheterosexual sexual orientation that were identical to that found in this sample for men (3.1%) but somewhat lower for women (1.5%). It seems unlikely that we have underestimated the population prevalence of nonheterosexual sexual orientation. Third, we were able to explicitly test for the equal-environment assumption, albeit with low statistical power. We found no evidence that more similar environmental experiences in monozygotic than in dizygotic twins contributed to the greater resemblance for sexual orientation in the monozygotic pairs.
However, this sample also has five potentially important limitations. First, the assessment of the complex phenotype of sexual orientation with a single item is far from ideal. Sexual orientation involves, at a minimum, dimensions of sexual fantasy, attraction, and behavior, none of which was explicitly captured by our single item. Second, despite the reasonable sample sizes, the relative rarity of nonheterosexual sexual orientation in general population samples results in quite low statistical power. For example, the 95% confidence intervals (CIs) around the estimates of a2 in Table 1 all include zero. If we examine the AE submodel (which provides the best fit for three of the four analyses), we gain in the accuracy of parameter estimation. However, even here, the 95% CIs for heritability range widely—for example, from 31% to 89% for monozygotic plus same-sex dizygotic twins. Third, our analytic method did not account for the correlated observations in families with more than two siblings. We reran the analysis of all dizygotic pairs plus nontwin sibling pairs in a newly available option of Mx that corrected for the correlational structure and obtained estimates from the full model (a2=0.47, c2=0.20, e2=0.34) that were reassuringly similar to those obtained in our initial analyses. Fourth, the small number of cases of nonheterosexual sexual orientation made it impossible to examine gender differences in the causes of sexual orientation with any statistical power. Qualitative comparisons indicated that the concordance rate for nonheterosexual sexual orientation was higher in the female-female pairs than in the male-male pairs among the monozygotic twins (four of nine, or 44.4%, versus two of 10, or 20.0%) and for the dizygotic twins plus nontwin sibling pairs (eight of 28, or 28.6%, versus four of 28, or 14.3%). The evidence that pair resemblance for sexual orientation is greater in same-sex than in opposite-sex pairs suggests that the familial factors that influence sexual orientation may not be the same in males and females. Fifth, our overall cooperation rates were only moderate for the twin sample and even lower for the nontwin sibling pairs. However, our evidence for heritable influences on sexual orientation was somewhat stronger for analyses that included only twins than for those that also included siblings. This would not be the expected pattern if the evidence for genetic effects on sexual orientation was due to cooperation bias, indirectly arguing that the key finding is substantive rather than due to a methodological artifact.