Benzodiazepines are frequently prescribed for the pharmacological management of general symptoms of anxiety
(1,
2). However, the achievement of successful benzodiazepine discontinuation after prolonged treatment is often difficult, even for patients maintained on moderate to low therapeutic doses
(3). Management of benzodiazepine discontinuation in long-term benzodiazepine users includes gradual rather than abrupt discontinuation
(4,
5), use of cognitive therapy
(6,
7), and the concomitant prescribing of pharmacological agents such as carbamazepine
(8), trazodone
(9,
10), and valproate
(10).
The present study was designed to assess the possible usefulness of imipramine and buspirone in the management of benzodiazepine discontinuation. The antidepressant imipramine was chosen because it possesses both antidepressant and anxiolytic properties
(11,
12). The anxiolytic buspirone was chosen because it demonstrates at lease some antidepressant effects in addition to its anxiolytic properties
(13,
14). We hypothesized that treatment of underlying anxious and subsyndromal depressive symptoms would ease withdrawal and help patients discontinue benzodiazepine intake.
Method
Subjects
All patients were treated at the Psychopharmacology Research Unit of the University of Pennsylvania. Patients were recruited by physician referrals and notices in local media. The study was approved by the institutional review board of the University of Pennsylvania. After complete description of the study to the subjects, written informed consent was obtained.
To be enrolled in the program, patients were required to have a diagnosis of generalized anxiety disorder according to DSM-III-R, to be at least 21 years old, and to have been taking diazepam, lorazepam, or alprazolam in therapeutic doses continuously for the past 12 months (5 mg diazepam was considered equivalent to 1 mg lorazepam and 0.5 mg alprazolam). Six patients who were taking more than one benzodiazepine were switched to one of the three study benzodiazepines before entering the study. Each patient’s study eligibility was confirmed by means of a screening medical history, a physical examination, and laboratory tests, including CBC, blood chemistries, urinalysis, test of benzodiazepine plasma level, and urine drug screens.
Thirteen patients received a current secondary psychiatric diagnosis, including major depressive disorder for five patients, dysthymia for four patients, and panic disorder for four patients. The 13 patients with a secondary diagnosis were evenly distributed between the imipramine, buspirone, and placebo groups (χ2=1.73, df=2, n.s.). Patients with a primary panic disorder diagnosis were excluded from this report.
Study Design
During the screening phase, patients were kept on a stable dose of their benzodiazepine within the therapeutic range for 2–4 weeks. They were then assigned to double-blind treatment with either imipramine, buspirone, or placebo, while the daily benzodiazepine intake was not altered. Four weeks later, patients entered a taper phase that lasted 4–6 weeks. During the taper phase, daily benzodiazepine intake was reduced at a rate of approximately 25% per week. The taper phase was followed by a 5-week benzodiazepine-free phase during which the patient’s clinical status in the initial period without benzodiazepines was prospectively assessed. Double-blind study treatment was continued for the first 3 weeks of the benzodiazepine-free phase; placebo was substituted, single blind, for imipramine and buspirone for the final 2 weeks. Patients were seen weekly. After participation in the benzodiazepine discontinuation program, patients were returned to the care of their family physicians. They returned to the study clinic for follow-up at 3 months posttaper, and a follow-up clinic visit or telephone interview was conducted at 12 months. Both follow-ups assessed benzodiazepine status, use of psychiatric treatment, and symptom severity.
Benzodiazepine and Imipramine Plasma Assays
Benzodiazepine status was confirmed by plasma benzodiazepine determinations, which were performed at each study visit. Diazepam and desmethyldiazepam concentrations were quantitated by gas chromatography with electron capture detection
(15). Levels of lorazepam or alprazolam in plasma were quantified by using similar methods
(16,
17). The assay techniques had a sensitivity limit of 1–2 ng/ml and a coefficient of variation for identical samples that did not exceed 10%. Regardless of the specific benzodiazepine assessed, each plasma level determination also included an overall plasma benzodiazepine screen to allow detection of any benzodiazepine taken that was unreported. Imipramine and desmethylimipramine plasma levels were assessed in a subsample of 12 patients
(18).
Study Medication
Medication was prepared double blind in identical capsules containing either 5 mg buspirone, 25 mg imipramine, or placebo. Compliance with prescribed doses was assessed both by pill count and a daily diary that was reviewed with the patient at each study visit. Every attempt was made to achieve a daily dose of at least six capsules (i.e., 150 mg of imipramine and 30 mg of buspirone) at the end of 2 weeks. After 4 weeks of treatment, mean daily intake for imipramine, buspirone, and placebo was 7.2 capsules (SD=2.6) (180 mg), 7.6 capsules (SD=2.5) (38 mg), and 8.7 capsules (SD=2.4), respectively (F=2.79, df=2, 72, p<0.10), taken in divided doses.
Clinical Assessments
Psychiatric diagnosis was assessed by a research psychiatrist who used a semistructured interview based on DSM-III-R diagnostic criteria. Discontinuation symptoms were assessed with an empirically derived 38-item physician-rated checklist of withdrawal symptoms (physician-rated withdrawal checklist)
(4), and the patient completed the withdrawal cluster derived from the HSCL
(19), as described by Covi et al. (Covi withdrawal checklist)
(20). Both checklists inquired about symptoms present during the past week. The Hamilton Anxiety Rating Scale, the Hamilton Depression Rating Scale, and the patient-rated HSCL were used to provide measures of anxiety and depression. Possible responses to HSCL items ranged from 1 (“not ill”) to 4 (“extremely ill”)
(20).
Primary Outcome Measures
The primary outcome measures were 1) whether the taper was successful and 2) the severity of symptoms of benzodiazepine discontinuation. A successful taper was defined as achievement of a benzodiazepine-free state and maintenance of that state for 12 weeks posttaper.
Statistical Analysis
All analyses were performed with the SAS statistical software package
(21). Chi-square tests with Yates’s correction or Fisher’s exact test were performed on data for dichotomous variables, and analyses of variance or covariance were used for actual scores and change scores. All results were interpreted conservatively as two-tailed, with significance level set as p<0.05. Individual group comparisons used the least square means method. Data presented in tables and figures were based on actual scores. A logistic regression analysis was performed for nine potential predictors of benzodiazepine-free outcome.
Results
Demographic and Clinical Characteristics
No significant demographic or clinical differences were noted between patients in the three treatment groups. For the combined patient group of 107 patients, mean age was 48 years (SD=14, range=22–77). Forty-eight patients (44.9%) were female, 73 (68.2%) had more than a high school education, 22 (20.6%) smoked, and 36 (33.6%) had previously used recreational drugs, primarily tetrahydrocannabinol; mean daily coffee intake was 3.4 cups of coffee (SD=5.1). The patients’ mean benzodiazepine plasma levels at baseline were 932 ng/ml (SD=715) for diazepam plus desmethyldiazepam, 29 ng/ml (SD=16) for lorazepam, and 26 ng/ml (SD=20) for alprazolam. Mean daily benzodiazepine intake was 15.9 mg/day (SD=10.2) of diazepam, 2.5 mg/day (SD=1.7) of lorazepam, and 2.1 mg/day (SD=1.8) of alprazolam. Most patients (N=97, 90.7%) had previously attempted to discontinue benzodiazepine intake, with an average number of 3.4 attempts (SD=5.5). The patients had been taking a benzodiazepine for a mean of 102 months (SD=92, range=11–372). Only 24.3% (N=26) reported a satisfactory therapeutic response to their benzodiazepine therapy. Mean scores for measures of anxious and depressive symptoms at screening were 12.5 (SD=6.3) on the Hamilton anxiety scale, 11.6 (SD=6.5) on the Hamilton depression scale, 2.12 (SD=0.67) on the HSCL anxiety factor, and 1.95 (SD=0.62) on the HSCL depression factor.
Attrition During Pretreatment
Of the 107 patients who entered the program, 32 patients did not complete the pretaper treatment phase, leaving 75 patients to enter the taper phase. These two patient groups did not differ in any baseline demographic or clinical measures. For example, the mean benzodiazepine dose at screening was 19.2 mg diazepam equivalents (SD=14.6) for the dropouts and 16.3 mg diazepam equivalents (SD=14.0) for the patients who entered the taper phase (F=0.74, df=1, 105, n.s.); the two groups’ mean Hamilton anxiety scale scores at screening were 13.0 (SD=5.6) and 13.3 (SD=6.6), respectively (F=0.27, df=1, 105, n.s.), and the mean lengths of time the two groups had taken benzodiazepines were 86 months (SD=74) and 109 months (SD=98), respectively (F=1.44, df=1, 105, n.s.).
The dropouts also did not differ from the patients who entered the taper phase in assignment to the three treatment groups (χ2=2.05, df=2, n.s.) or in type of benzodiazepine used at baseline (χ2=1.60, df=2, n.s.). The main reasons patients gave for dropping out during the pretreatment phase were having changed their mind about participating (N=16), adverse events (N=4 in the imipramine group, N=4 in the buspirone group, N=6 in the placebo group), and increased anxiety (N=2). A total of 23 patients in the imipramine group, 28 in the buspirone group, and 24 in the placebo group entered the taper phase.
Plasma Levels
Benzodiazepine plasma levels were extremely stable from baseline to the end of the 4-week pretaper treatment period and were within the expected therapeutic range
(22,
23). Benzodiazepine half-life determined how long plasma levels could be detected after completion of the taper (1 week for lorazepam and alprazolam and 4 weeks for diazepam and desmethyldiazepam). None of the patients who reported having discontinued benzodiazepine at either 5 weeks or 12 weeks had detectable benzodiazepine plasma levels. For a subsample of 12 patients, mean imipramine and desipramine plasma levels at the beginning of the taper were 81 ng/ml (SD=56) and 45 ng/ml (SD=38), respectively.
Adverse Events
The percentage of patients reporting treatment-emergent events while taking imipramine, buspirone, or placebo during the pretaper phase (when reporting of adverse events was not influenced by withdrawal symptoms) was highest for those receiving imipramine and lowest for those receiving placebo. Statistically significant differences between the imipramine, buspirone, and placebo groups (N=26, N=43, and N=34, respectively) were found for six adverse events: dry mouth (N=21 [80.8%], N=10 [23.3%], and N=8 [23.5%], respectively) (χ2=27.2, df=2, p<0.001); constipation (N=8 [30.8%], N=4 [9.3%], and N=4 [11.8%], respectively) (χ2=6.2, df=2, p<0.04); sedation (N=17 [65.4%], N=12 [27.9%], and N=13 [38.2%], respectively) (χ2=9.6, df=2, p<0.01); lightheadedness (N=9 [34.6%], N=19 [44.2%], and N=5 [14.7%], respectively) (χ2=7.7, df=2, p<0.03); insomnia (N=8 [30.8%], N=11 [25.6%], and N=2 [5.9%], respectively) (χ2=6.8, df=2, p<0.04); and vivid dreams (N=5 [19.2%], N=1 [2.3%], and N=2 [5.9%], respectively) (χ2=6.7, df=2, p<0.04). Patients who took imipramine primarily reported dry mouth, blurred vision, constipation, tremor, sedation, and insomnia. Patients who took buspirone primarily reported lightheadedness and insomnia.
Symptom Reduction During the Pretreatment Phase
Both imipramine and buspirone caused slightly more symptom reduction than placebo during the 4-week pretaper treatment period in the 75 patients who entered the taper phase. For example, the mean decrease from baseline in the Hamilton anxiety scale score was 3.5 (SD=5.5) for patients who received imipramine and 3.0 (SD=3.8) for patients who received buspirone, but only 0.1 (SD=2.9) for patients who received placebo (F=3.77, df=2, 67, p<0.03). The mean decrease from baseline in the Hamilton depression scale score in the three groups was 4.0 (SD=5.6), 3.0 (SD=5.3), and 0.1 (SD=2.9), respectively (F=3.24, df=2, 67, p<0.05).
Benzodiazepine Discontinuation Symptoms
The severity of withdrawal symptoms was assessed weekly with the physician-rated withdrawal checklist, the Hamilton anxiety scale, and the Covi withdrawal checklist. Measures of change in withdrawal symptom severity from the pretaper baseline to peak severity of symptoms during the taper phase indicated that symptom severity increased in patients in all three groups, but that the patients who received imipramine reported significantly more severe symptoms than the patients who received either buspirone or placebo (mean change in severity as measured with the physician-rated withdrawal checklist=16.6 (SD=13.3) for the imipramine group, 8.9 (SD=7.5) for the buspirone group, and 10.4 (SD=10.6) for the placebo group) (F=3.76, df=2, 65, p<0.03). Thus, no significant beneficial effects of either buspirone or imipramine on benzodiazepine withdrawal severity were found. Similar results were obtained when change in the three groups’ scores on the Hamilton anxiety scale, the HSCL, and the Covi withdrawal checklist were compared. Pearson product-moment correlations between mean scores on the physician-rated withdrawal checklist and the Hamilton anxiety scale and between mean scores on the physician-rated withdrawal checklist and the Covi withdrawal checklist at peak severity of withdrawal symptoms were 0.91 (df=71, p<0.001) and 0.81 (df=70, p<0.001), respectively.
Success of the Benzodiazepine Taper
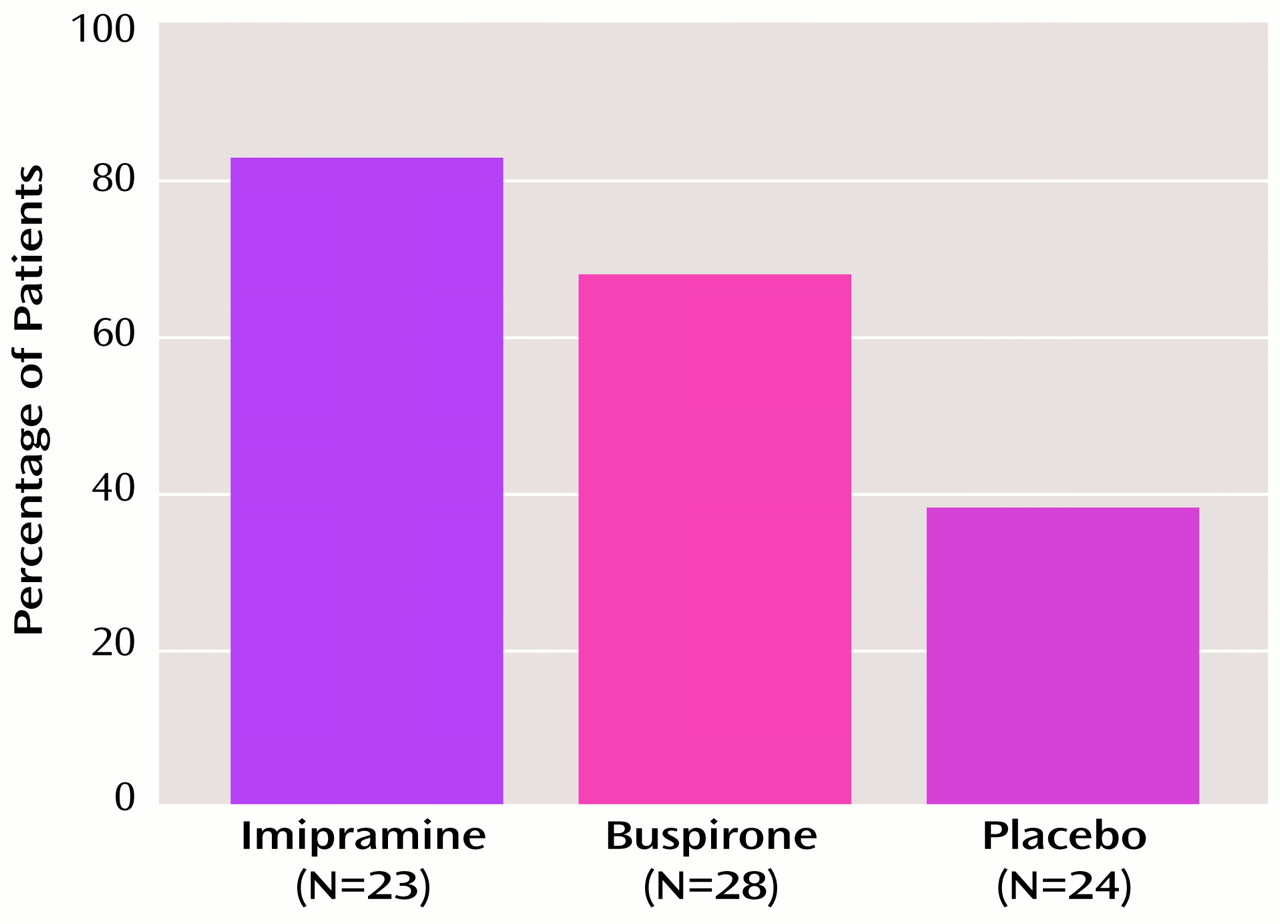
The success of the benzodiazepine taper was measured by benzodiazepine status at 3 months after benzodiazepine discontinuation. The results are shown in
Figure 1. The results include data for five patients who were not available for 3-month follow-up and whose 5-week posttaper status was carried forward (two were benzodiazepine free at 5 weeks, and three showed evidence of benzodiazepine use). Significantly more patients in the imipramine group (82.6%) than in the placebo group (37.5%) achieved benzodiazepine-free status (χ
2=8.14, df=1, p<0.01, N=47). More patients in the buspirone group (67.9%) than in the placebo group achieved benzodiazepine-free status; although the difference was not statistically significant, it approached significance (χ
2=3.65, df=1, p<0.06, N=52). A logistic regression analysis was conducted for nine potential predictors of 3-month taper success. Of the nine potential predictors, eight had Pearson product-moment correlations with taper outcome at p<0.10. One additional predictor, benzodiazepine half-life, was entered in the analysis because of its potential clinical interest. Neither benzodiazepine half-life nor four other variables (peak severity of withdrawal symptoms, change in Hamilton anxiety scale score during the pretreatment phase, prior recreational drug use, and buspirone use) entered the regression equation at significance levels of p≤0.10.
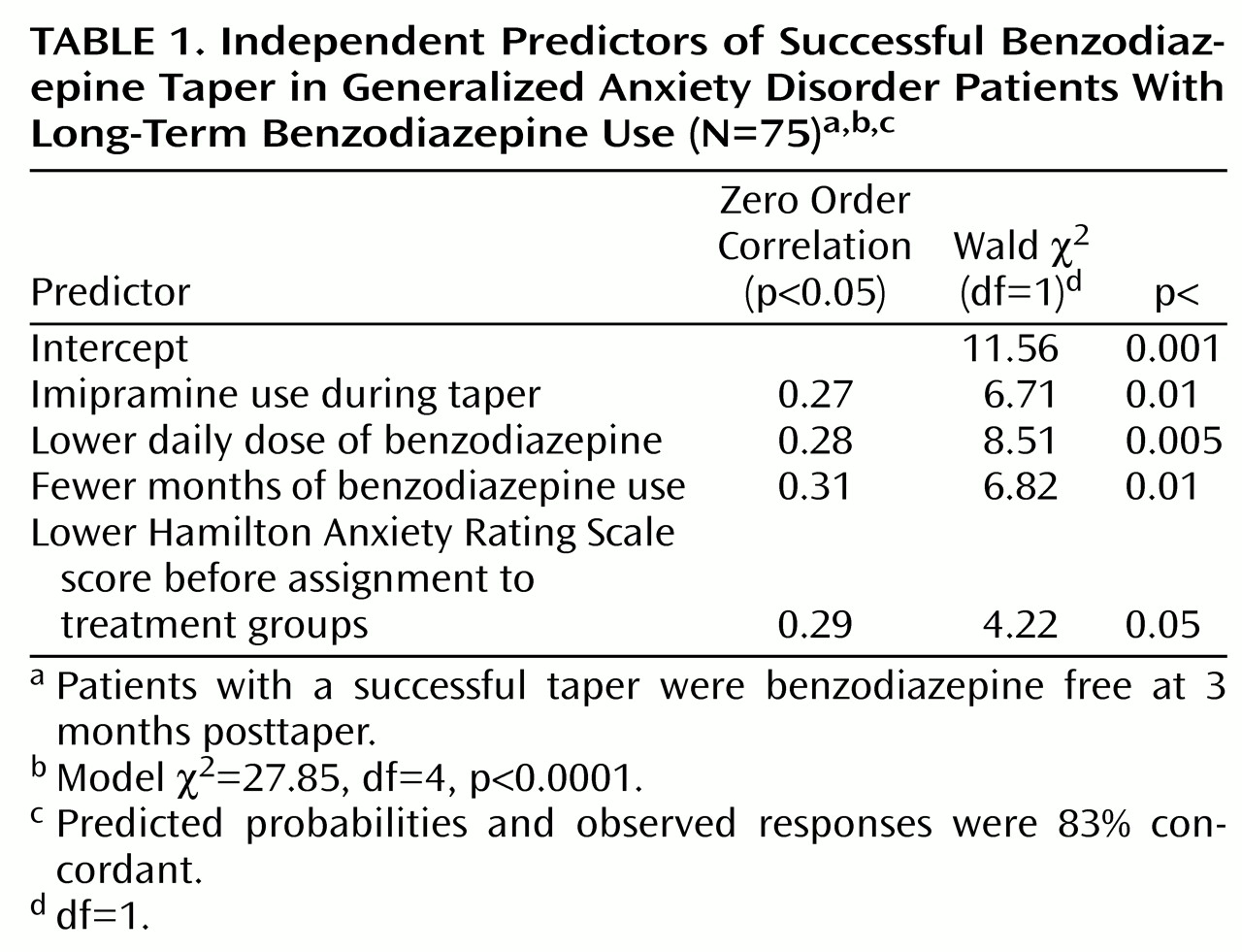
Table 1 shows the variables that independently predicted taper success at a statistically significant level. The variables, shown in order of selection by the regression procedure, were imipramine use, lower daily benzodiazepine intake, fewer months taking benzodiazepines, and lower baseline level of anxiety symptoms.
At 3-month follow-up, patients who did not have a successful taper had significantly reduced their daily benzodiazepine intake, from 21 mg/day diazepam equivalents (SD=17) at baseline to 15 mg/day diazepam equivalents (SD=15) at 3-month follow-up (t=2.71, df=18, p<0.02, N=20).
12-Month Follow-Up
Thirty-two of the 40 patients (80.0%) who were benzodiazepine free at 3 months were also benzodiazepine free at 12 months, compared to only seven of the 17 patients (41.2%) who were still taking benzodiazepines at 3 months (χ
2=8.32, df=1, p<0.005). Thus, for the 57 patients for whom data on benzodiazepine intake at the 12-month follow-up were available, 39 (68.4%) were benzodiazepine free and 18 (31.6%) were still taking benzodiazepines. Three of the four significant predictors of 3-month posttaper benzodiazepine-free status (i.e., imipramine use, adjusted benzodiazepine dose, and months taking benzodiazepines, but not the Hamilton anxiety scale score at baseline) still had some predictive power at 12 months, but each only at the 10% level (model χ
2=10.46, df=4, p<0.04).
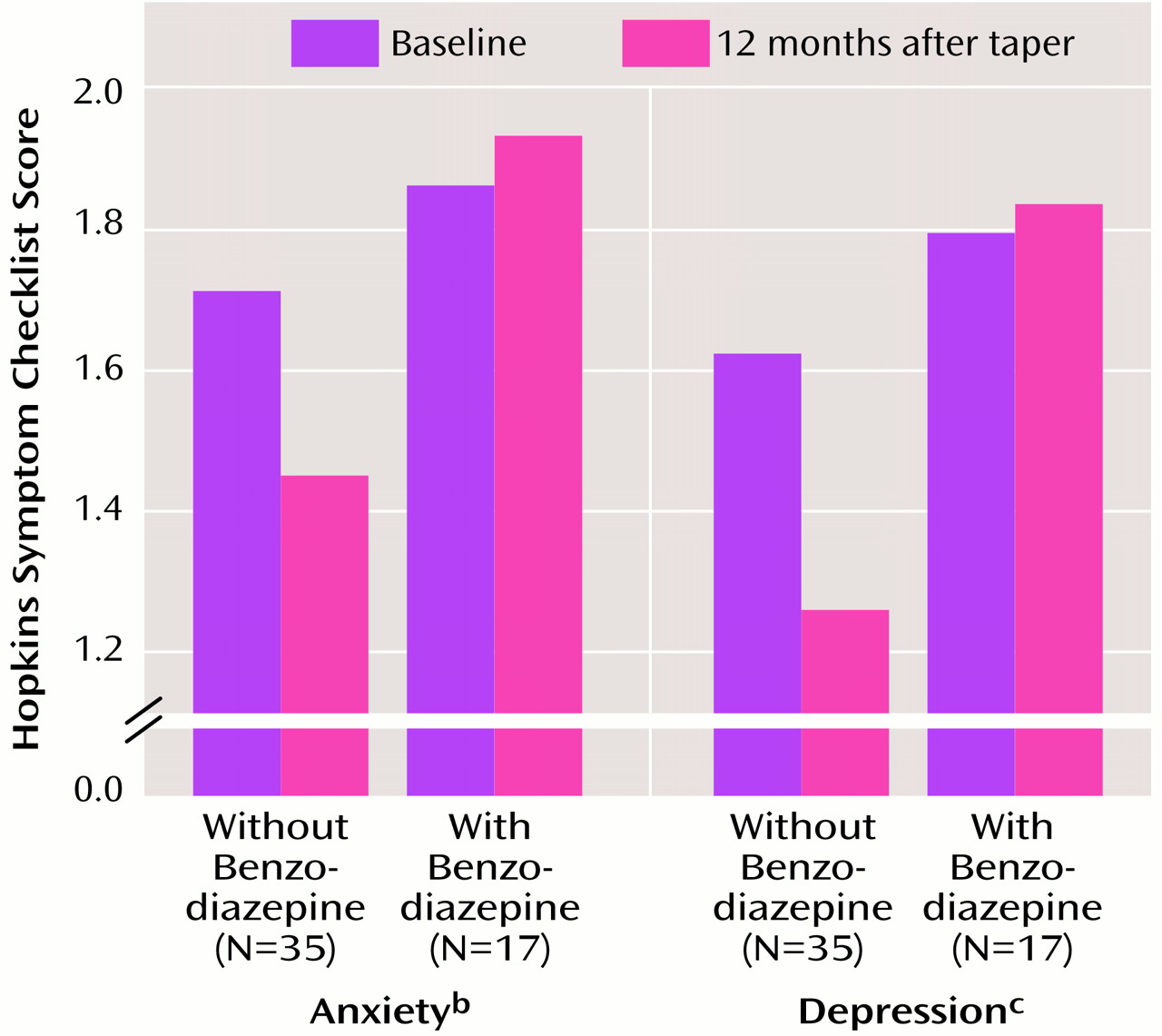
Figure 2 gives results for the HSCL anxiety and depression factors for patients who were benzodiazepine free and those still taking benzodiazepines at 12 months after completion of the taper phase. Clearly, benzodiazepine-free patients reported significantly lower levels of anxiety and depression than patients still taking benzodiazepine, but their baseline scores were also slightly lower than those of the patients who were still taking benzodiazepines, although not significantly lower. In fact, the 12-month posttaper scores of the benzodiazepine-free patients closely approximated scores reported by nonpsychiatric patients
(24).
Discussion
We report results from a large double-blind, placebo-controlled study of the pharmacological management of benzodiazepine discontinuation in long-term benzodiazepine users with generalized anxiety disorder (mean duration of benzodiazepine use >8 years). As hypothesized, 4 weeks of aggressive pretaper treatment with imipramine or buspirone but not placebo yielded modest but statistically significant levels of reduction of symptoms of anxiety and depression.
This modest reduction in symptoms of anxiety and depression translated into successful taper outcome but not into a reduction of the symptoms of benzodiazepine discontinuation. The high rates of adverse effects observed with imipramine may have contributed to our negative findings for withdrawal severity. It is of interest that other concomitant treatments investigated for use in facilitating benzodiazepine taper proved similarly ineffective in reducing benzodiazepine withdrawal severity
(10,
25–
27). One may speculate that long-term benzodiazepine users are especially sensitive to the noradrenergic effects of imipramine, and also to that of buspirone’s major metabolite, 1-(2-pyrimidinyl)-piperzine
(28).
Pretreatment and concomitant use of imipramine during taper, however, caused a significant increase in the taper success rate, and this effect was maintained even after adjusting for daily benzodiazepine intake, duration of benzodiazepine therapy, and level of anxiety symptoms at baseline. Of the patients who received imipramine, 82.6% were benzodiazepine free at 3 months posttaper, compared to 67.9% of patients who received buspirone and only 37.5% of patients who received placebo. The positive results with imipramine may be related not only to its antidepressant and anxiolytic activity but also to its clinical effect on neurochemical substrates implicated in benzodiazepine withdrawal symptoms
(29). The findings for buspirone support earlier reports of its inability to facilitate benzodiazepine taper even when patients were aggressively treated as in the present study
(30). Its increased but nonsignificant effect in facilitating taper finds support, however, in a study by Delle Chiaie et al.
(31), who reported that buspirone facilitated taper in patients taking lorazepam for 3 months or less. Finally, the inability of Tyrer et al.
(25) to observe any beneficial taper effect for dothiepin, a tricyclic antidepressant, might be related to differences in antidepressant and anxiolytic properties between imipramine and dothiepin, or to differences in study design.
The observed lack of correlation between withdrawal severity and successful taper outcome (r=0.05) can most likely be explained by our recent observation that passive-dependent personality characteristics contribute significantly to taper failures early in the taper, when withdrawal symptoms are still minimal, but not to late taper failure
(32).
The 12-month follow-up data are of clinical interest from several points of view. First, as in an earlier study
(33), most patients (80.0%) who successfully tapered their benzodiazepine remained benzodiazepine free at 12-month follow-up. Second, 41.2% of patients who were not able to complete the taper also were benzodiazepine free. Thus, participation in a benzodiazepine discontinuation program, even if initially unsuccessful, led to a decrease in daily benzodiazepine intake and allowed about 40% of unsuccessful taper patients to be benzodiazepine free 12 months later. Third, from the clinician’s point of view, probably the most interesting finding was that patients who were benzodiazepine free at the 12 month follow-up reported significantly lower levels of anxiety and depression symptoms, adjusted for baseline symptoms, than the patients who continued to take benzodiazepines.
Two alternative explanations for this finding are offered. Before the taper, some long-term benzodiazepine users may have used the medication to treat a constant state of mild withdrawal rather than to treat a current anxiety condition. However, for other patients, benzodiazepine therapy may have fulfilled a clinical purpose. In fact, at the 12-month follow-up, seven patients who were benzodiazepine free and four patients who continued to take benzodiazepines were taking tricyclics or selective serotonin reuptake inhibitors (SSRIs). These 11 patients had higher anxiety levels at follow-up than the other 41 patients for whom 12-month follow-up data on anxiety level were available, although the differences were not significant (among the patients who were benzodiazepine free at 12 months, HCSL anxiety factor mean=1.84 [SD=0.62] for the seven patients who were taking tricyclics or SSRIs and mean=1.35 [SD=0.43] for the 28 patients who were not taking tricyclics or SSRIs (F=3.18, df=1, 32, p<0.09); among the patients who were taking benzodiazepine at 12 months, mean=2.21 [SD=0.73]) for the four patients who were taking tricyclics or SSRIs and mean=1.84 [SD=0.35] for the 13 patients who were not taking tricyclics or SSRIs (F=2.54, df=1, 14, p<0.14).
This study had some limitations that should be considered in interpreting the findings. First, patients who entered the benzodiazepine discontinuation program had been taking benzodiazepines at therapeutic doses for an average of more than 8 years, and almost all of them had previously attempted unsuccessfully to taper benzodiazepine on several occasions. Thus, the study group should be considered rather treatment resistant. Second, the study patients entered the taper program with mild to moderate levels of anxiety and depression symptoms, and only 24.3% had reported a satisfactory therapeutic response to their benzodiazepine treatment before the taper. In contrast, patients in the community who receive benzodiazepine therapy as part of a medically supervised regimen prescribed for a specific period of time and who have not failed previous taper attempts may be expected to experience less severe withdrawal symptoms and a higher benzodiazepine-taper success rate, not only with imipramine but also with buspirone. Third, the results pertain only to long-term benzodiazepine users with a diagnosis of generalized anxiety disorder and thus do not provide data on benzodiazepine discontinuation for long-term benzodiazepine users with other psychiatric diagnoses. Fourth and finally, the tricyclic antidepressant imipramine, which was used in this study, caused adverse events that were rather disturbing to some patients, as reflected in the high rates of benzodiazepine discontinuation symptoms in patients who received imipramine, compared to those who received buspirone and placebo. Therefore, it would be of interest to test an SSRI with and without noradrenergic activity in this population. Substitution of an SSRI for imipramine could lead to a reduction of both adverse events and benzodiazepine withdrawal symptoms.