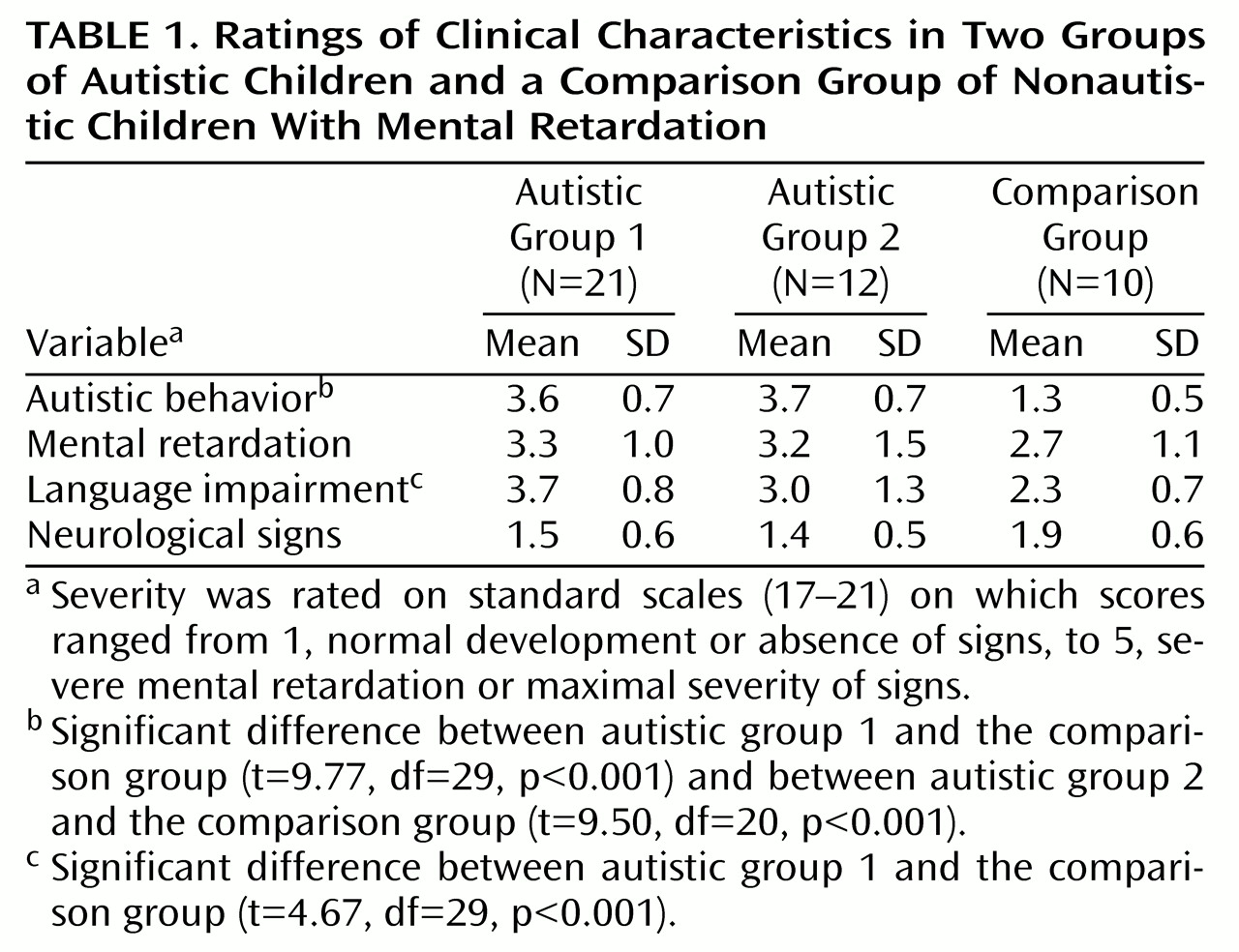
Progress in understanding the neural basis of childhood autism requires clear and reliable data indicating specific neuroanatomical or neurophysiological abnormalities. Localized structural and functional brain correlates of autism have yet to be established
(4,
5). Previous brain imaging studies performed in autistic patients have reported abnormalities such as a larger total brain volume
(6,
7), cerebellar abnormalities
(8,
9), a smaller number of cortical metabolic correlations
(10), and a delayed pattern of cortical metabolic maturation
(11). Taken together these results are consistent with an anomalous pattern of brain organization in autism, but none of them fully account for the full range of autistic symptoms. Furthermore, most functional imaging studies have reported normal or near normal regional brain metabolism and cerebral blood flow
(12–
15). Yet, localized cerebral cortex abnormalities may have been overlooked with first-generation functional brain imaging techniques.
In the study reported here, we address this issue with up-to-date functional brain imaging methods. We used positron emission tomography (PET) and a voxel-based whole brain analysis to search for abnormalities in regional cerebral blood flow (rCBF), an indirect marker of neural activity, in children with primary autism. Twenty-one autistic children were compared to 10 nonautistic children. The results led us to perform an extension study in a separate group of 12 autistic children.
Results
Initial Study—Group Analysis
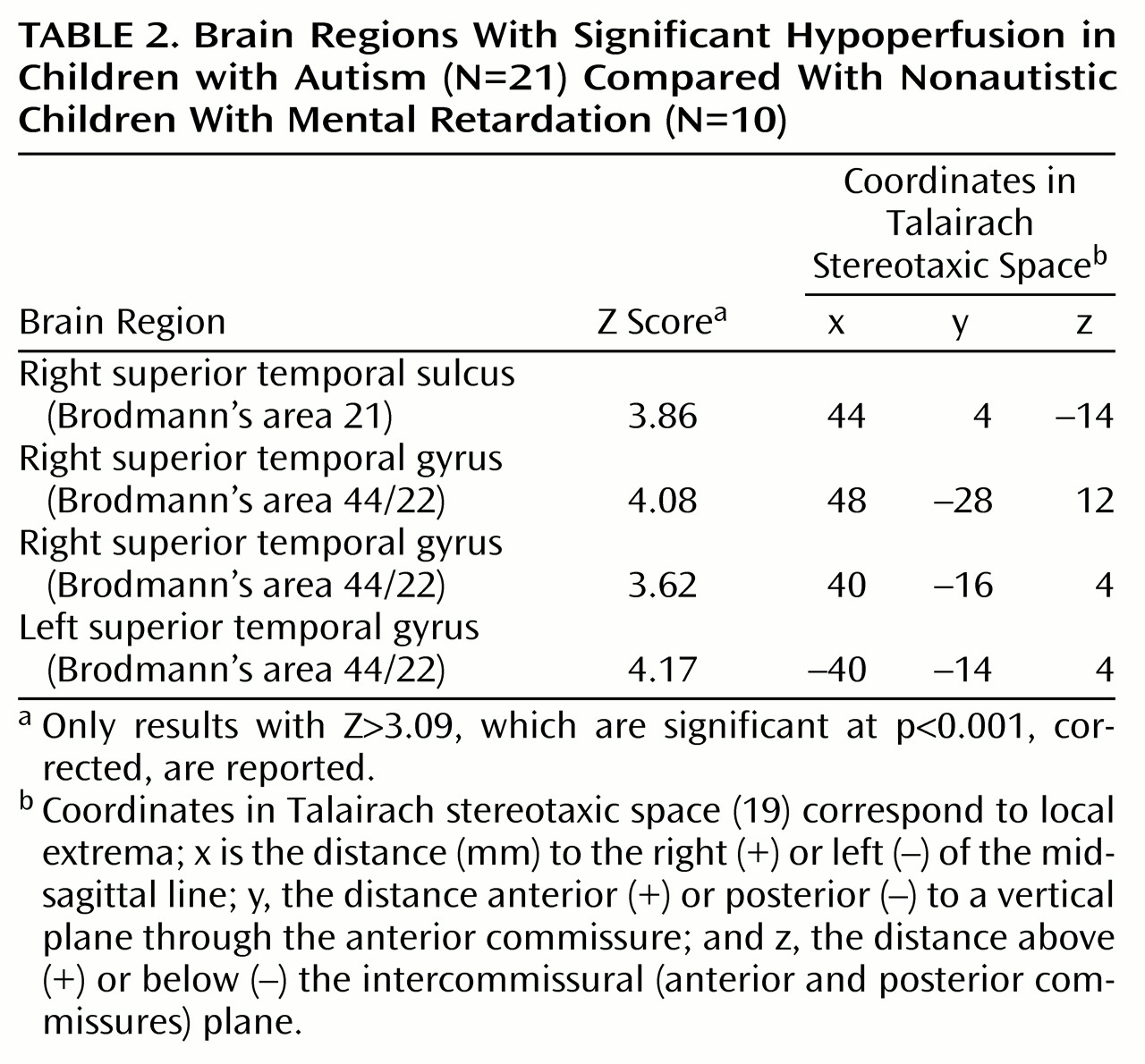
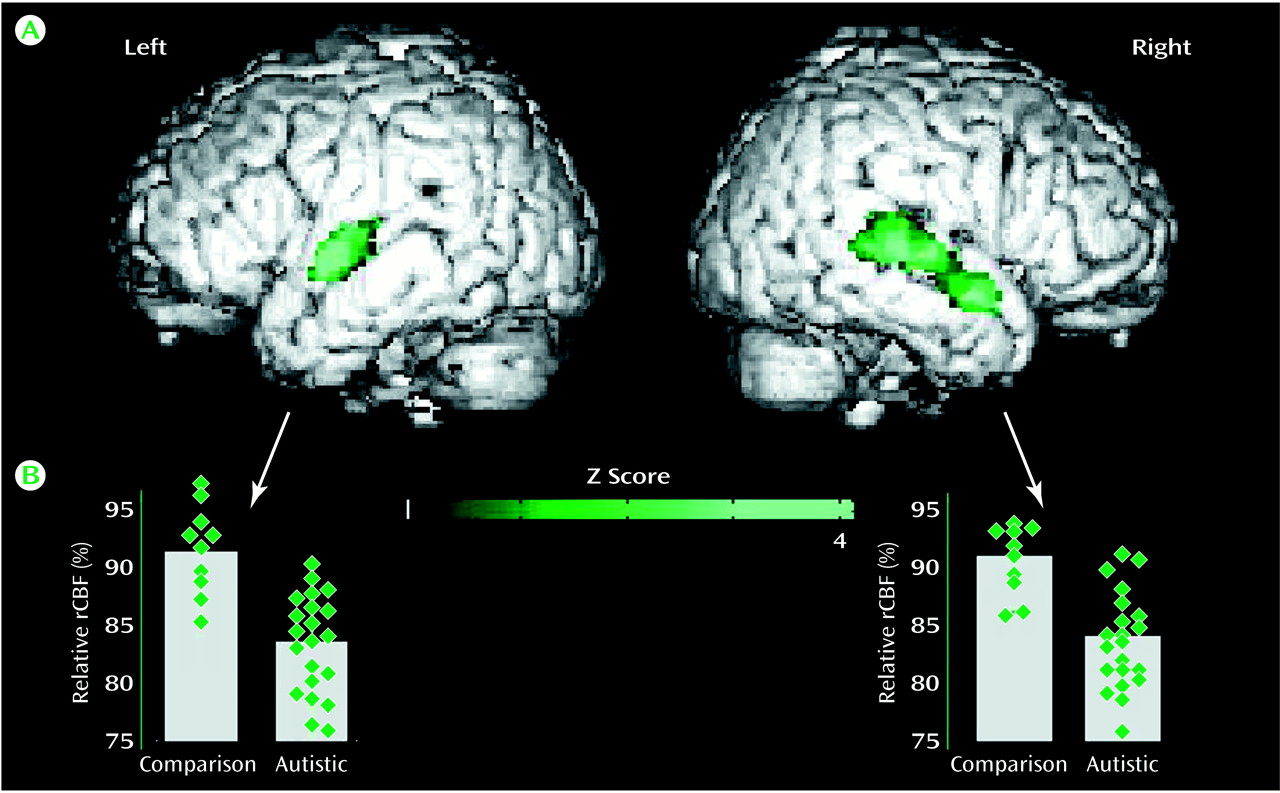
SPM group analysis revealed a significant hypoperfusion (t=4.12–4.94, df=29, p<0.001; corrected p<0.05) in the autistic group in four foci localized in the temporal lobe, three in the right side, and one in the left (
Figure 1). Regions of maximal hypoperfusion were centered in the left superior temporal gyrus (Brodmann’s area 22/42), in the right superior temporal gyrus (Brodmann’s area 22/42), and in the right superior temporal sulcus (Brodmann’s area 21) (
Table 2). We did not find any hypoperfused cerebral regions in the mentally retarded comparison group.
The principal components analysis showed that the group effect (autistic versus comparison children) explained much more of the total variance of the data set (49.2%) than the effect of the different scanners used in the study (10.0%). In addition, within-scanner comparisons yielded results similar to those of the global analysis, although less statistically significant.
Initial Study—Individual Analysis
A significant temporal hypoperfusion (t=2.84–5.40, df=9, p<0.01) was detected in 16 of 21 autistic children (76.2% positive individual detection). The temporal hypoperfusion was bilateral in seven of 16 autistic children and was located on the right side in nine autistic children.
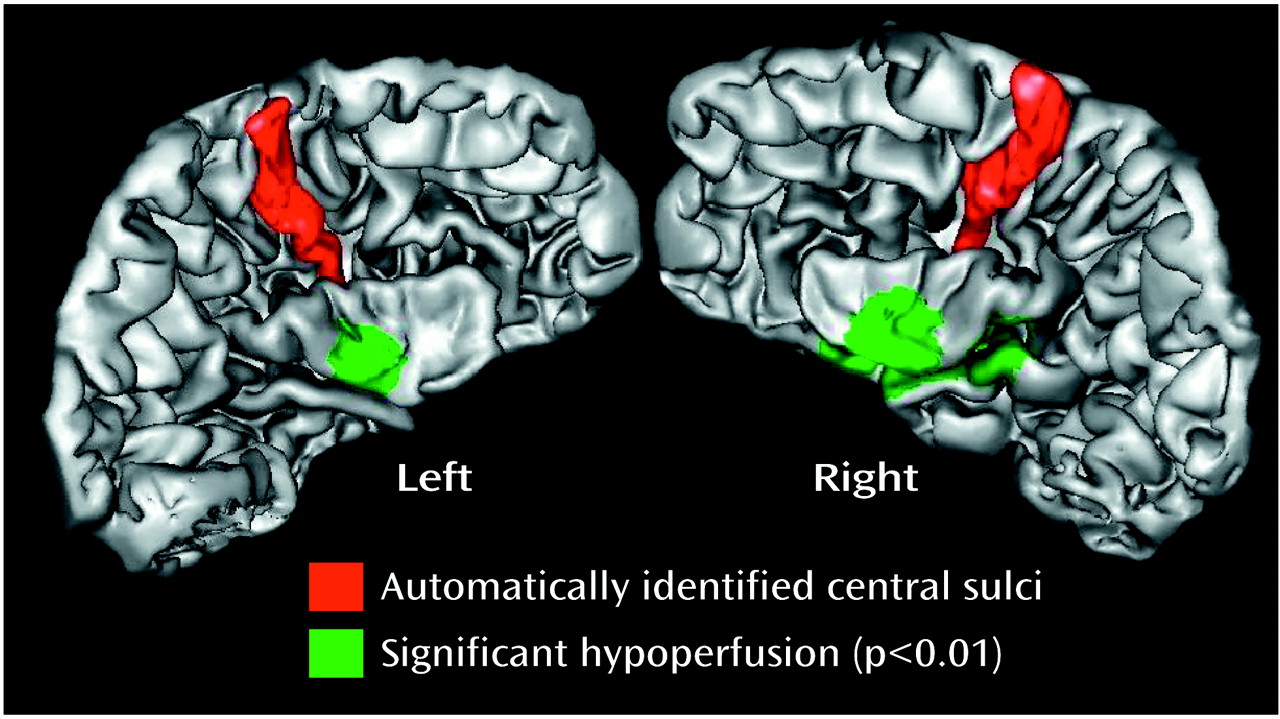
Figure 2 shows brain regions with significant hypoperfusion in an autistic child with bilateral temporal hypoperfusion. The exploratory analysis of extratemporal regions revealed abnormalities in five of the 21 autistic children: three of the children had an abnormality in the frontal region, one in the frontocerebellar region, and one in the occipital region.
Extension Study—Group Analysis
We found the same pattern of bitemporal hypoperfusion that was observed in the initial study. Four foci of hypoperfusion (t=4.65–5.74, df=20, p<0.001; corrected p<0.05) were detected. They were localized in the left and right superior temporal gyrus and in the right superior temporal sulcus, within millimeters of those found in the initial study.
Extension Study—Individual Analysis
A significant temporal hypoperfusion (t=2.92–8.36, df=9, p<0.01) was detected in nine of the 12 autistic children (75.0% positive individual detection). The temporal hypoperfusion was bilateral in two of the nine autistic children and was located on the right side in the remaining seven autistic children. The exploratory analysis of extratemporal regions revealed abnormalities in four of the 12 autistic children: two children had an abnormality in the frontal region, one in the frontocerebellar region, and one in the occipital region.
Clinical Correlations
To investigate the relationships between the pattern of temporal hypoperfusion and the clinical profile of the autistic children, we divided the autistic children into three groups: 1) those with a bilateral temporal hypoperfusion (N=9), 2) those with a right-side temporal hypoperfusion (N=16), and 3) those without temporal hypoperfusion (N=8). The mean age of these three subgroups was not significantly different. The three groups’ mean scores for autistic behavior were also very similar (mean=3.6 [SD=0.7], mean=3.6 [SD=0.8], and mean=3.9 [SD=0.6], respectively), and no significant difference was observed in the three groups’ scores for language impairments. The language score ranged from 1 (absence of impairment) to 5 (severe impairment) in the autistic group with bitemporal hypo-perfusion, from 2 (minor deficits) to 5 in the group with right temporal hypoperfusion, and from 2 to 5 in the group without temporal hypoperfusion (mean=3.6 [SD=1.1], mean=3.1 [SD=0.9], and mean=3.9 [SD=1.1], respectively). The mental retardation score was also similar between the three groups (mean=3.1 [SD=1.3], mean=3.1 [SD=1.3], and mean=3.8 [SD=1], respectively).
Discussion
We found marked bilateral hypoperfusion in the temporal lobes, centered in associative auditory and adjacent multimodal temporal cortex, in a group of autistic children. These findings were confirmed in a second group of autistic children and thus provide the first robust evidence for a localized dysfunction of cerebral cortex in school-aged children with primary autism. The temporal hypoperfusion could be detected on an individual basis in nearly 75% of the autistic children, with the current sensitivity of the imaging method.
These results contrast with those previously obtained with functional brain imaging in autistic patients at rest, most of which failed to find localized brain abnormalities in adult and school-aged autistic child
(12–
15). The differences could be explained by improvement in the spatial resolution of the imaging methods, roughly from 20 mm to 5 mm, between the current and earlier studies. In addition, the whole brain SPM analysis may be more appropriate than the region-of-interest method used in previous studies for detecting restricted foci of hypoperfusion in the temporal lobes.
Nevertheless, the results of the present study must be considered in the light of some methodological limitations. The first limitation is the lack, for ethical reasons, of rCBF values for normal children. Yet, inclusion of a comparison group of children with idiopathic mental retardation may be considered an advantage, since our findings cannot be attributed to the mental retardation associated with the autistic syndrome. Similarly, the findings cannot be attributed to language impairment, since temporal hypoperfusion was detected at a similar frequency in the autistic children with severe and mild language impairment. However, only 10 children were included in the comparison group, and our findings must be confirmed with a larger comparison group. The second limitation concerns the fact that rCBF measurements were performed during sleep. Since all subjects were studied under the same conditions, the findings reflect a true difference between autism and idiopathic mental retardation, but we cannot formally exclude that the possibility that the findings may have been different if measurements had been done when the subjects were awake. Finally, although the examinations were performed with two different PET scanners, the eigenimages of the principal components analysis confirmed that the difference between the autistic and nonautistic groups—and not the difference between scanners—accounted for most of the variance and explained the temporal hypoperfusion.
With these limitations in mind, we note that the finding of a bilateral temporal hypoperfusion extends to primary autism recent results suggesting a link between temporal lobe dysfunction and autistic behavior in children with neurological disorders. Indeed, autistic behavior has been associated with several clinical conditions resulting in temporal lobe pathology, such as epilepsy and herpes simplex encephalitis
(26–
30). Furthermore, two relatively recent neuroimaging studies have shown an association between temporal lobe abnormalities and the occurrence of secondary autism
(29,
30). In children with infantile spasms, the early finding of a bilateral temporal hypometabolism is strongly associated with later emergence of an autistic-like disorder
(29), whereas in children with tuberous sclerosis, there is a strong association between temporal lobe tubers and autistic syndrome
(30). Thus, early anatomical or metabolic abnormalities of the temporal lobes may lead to secondary autism. In addition, nonhuman primate experimental data have shown that neonatal medial temporal lesions cause behavioral disturbances strikingly similar to those seen in autistic children
(31).
A major challenge faced by all neurobiological theories of autism is to explain the variety of perceptive, cognitive, and affective deficits of autism. Although we do not currently have direct evidence, a theory based on temporal lobe dysfunction may address this challenge. First, autistic children have major sensory perceptual aberrations
(5), and the temporal lobe is thought to be central to the processing of numerous environmental signals that enter the nervous system through visual and auditory sense organs and to the organization of these signals into the structured patterns of neuronal activity forming the substrate of experiences that confer meaning to the world around us
(32). Furthermore, the temporal lobe dysfunction was centered in the auditory associative cortex and the superior temporal sulcus. Dysfunction of the auditory cortex may explain why young autistic child are so often initially misdiagnosed as deaf and why they often have severe communication impairments
(2,
5). Dysfunction of the superior temporal sulcus may indirectly explain the emotional and cognitive components of autism, since this multimodal association region is strongly connected with frontoparietal and limbic regions
(33,
34). These connections may also explain earlier brain imaging findings such as reduced functional parietofrontal connectivity and delayed frontal maturation
(10,
11).
In summary, voxel-by-voxel image analysis and rCBF PET studies demonstrated a well-localized functional abnormality in autistic children located bilaterally in the superior temporal cortex. The temporal hypoperfusion could be detected in nearly 75% of the autistic children in the study. These results suggest that the idiopathic autistic syndrome is related to a dysfunction of both left and right superior temporal regions and may be useful for guiding future research in developmental disorders.