A number of randomized controlled trials
(1–
3) have demonstrated that antipsychotic drugs belonging to the new generation of novel antipsychotics, such as risperidone and olanzapine, produce fewer adverse effects and offer, in the short term, better symptom relief than do conventional antipsychotics. However, there is much less information on the effectiveness under naturalistic conditions of the novel drugs in relation to conventional drugs and to each other.
A major indicator of drug effectiveness is prevention of relapse. Relapse in schizophrenia is a relative term reflecting symptomatic worsening or a behavior considered harmful to the patient and/or to society. Although far from perfect, the rate of relapse is often quantified by assessing the time from the last hospital discharge to the next hospitalization and the total number of hospitalizations per predetermined period. Some naturalistic follow-up studies have indicated that up to one-half of all stabilized patients are readmitted to the hospital within 1 year after discharge
(4). This high percentage is particularly worrisome since a high number of relapses is associated with poor long-term prognosis
(5), disruption of the patient’s life, and high societal costs.
In a recent study, Conley et al.
(6) compared the rehospitalization rates of patients discharged while taking risperidone or clozapine and found that at 24 months 87% of the clozapine-treated patients and 66% of the risperidone-treated patients remained in the community. Within the group of patients diagnosed with schizophrenia, the rates of rehospitalization for the two drugs were the same. In other studies
(7–
9), treatment with conventional antipsychotics was associated with 1-year rehospitalization rates ranging from 30% to 50%. Hogarty
(7) reported 1- and 2-year rehospitalization rates of 37% and 55%, respectively, for patients treated with conventional antipsychotics. In a meta-analysis of all published reports of rehospitalization, Weiden and Olfson
(8) estimated by survival curve analysis that 50% of patients treated with conventional antipsychotics will be readmitted within 1 year, and about 80% of patients by 2 years.
In the current study we compared the rehospitalization rates of all patients discharged while taking risperidone or olanzapine in Israel over a 2-year period to the rate for a group of patients treated with conventional antipsychotics. The study replicates and extends the design of Conley et al.
(6), who compared the rehospitalization rates of patients taking clozapine or risperidone who were discharged from six major public psychiatric facilities in Maryland. In addition, like Conley et al., we evaluated predictors of outcomes and identified risk factors associated with relapse within 24 months. Because of the unique reporting system in Israel, we are able to report on all patients discharged and readmitted over the period assessed.
Method
This report is based on a survey designed to monitor use of the novel antipsychotics risperidone and olanzapine in Israel and on data extracted from the National Psychiatric Hospitalization Case Registry. Olanzapine and risperidone are purchased for hospitals by the Ministry of Health. In an attempt to contain pharmacy costs, the Ministry of Health issued guidelines for the use of these drugs that limit their use to patients with a diagnosis of schizophrenia who have not responded to at least two conventional neuroleptics or who experienced severe side effects while taking conventional antipsychotics. To enforce the guidelines, the Ministry of Health requires all hospitals nationally to report to a drug registry the following information for each patient receiving novel antipsychotics: time of initiation, quarterly updates on doses, and time of therapy termination. The Ministry of Health reimburses the hospitals for the cost of the novel antipsychotics on the basis of this reporting; hence, there is an incentive for accurate reporting, and compliance is close to absolute.
Data from the drug registry were merged with data from the National Psychiatric Hospitalization Case Registry
(10), which includes hospitalization and demographic data. The registry is a complete listing of all psychiatric hospitalizations since 1950 regardless of type and auspices (public or private) of facility. A special department of the Ministry of Health verifies compliance in reporting, completion of forms, and consistency of information. The source of the data is the report of the treating board-certified psychiatrist, who is required by law to complete a form that is entered into the case registry for any admission to or discharge from a psychiatric bed in Israel. The demographic data are validated with the registry of the Ministry of Interior, which allows the addition of information on deaths to the data set. The study was judged to be exempt from the requirement for written informed consent by the Bar Ilan University human subjects committee.
All patients with schizophrenia discharged from any inpatient psychiatric facility in Israel while taking risperidone (N=268) or olanzapine (N=313) between Jan. 1, 1998, and Dec. 31, 1998, were followed up with respect to readmission until Jan. 1, 2000. Like Conley et al.
(6), we considered a successful hospitalization to be one in which the patient began with risperidone or olanzapine treatment and was discharged while taking that same drug. After discharge, all patients were given routine care, the overwhelming majority in community mental health centers, most of which were linked to the discharging hospital. No special care or therapy was provided for any of the patient groups. During the observation period of this study, 756 hospitalized schizophrenic patients nationally were treated with risperidone, of whom 268 (35.4%) were discharged during this period. Another 865 were treated with olanzapine, of whom 313 (36.2%) were discharged during this period.
The National Psychiatric Hospitalization Case Registry was also used to extract data on a group of patients who were treated during the same time period as those treated with the novel antipsychotics but who were treated with conventional antipsychotics. These patients were selected to be similar to the patients receiving novel antipsychotics. This was done by selecting patients who would have met the Ministry of Health guidelines for treatment with novel antipsychotics, which, as previously noted, are reserved for patients with a diagnosis of schizophrenia who have not responded to at least two conventional neuroleptics or who experienced severe side effects while taking conventional antipsychotics. We thus included only patients with a diagnosis of schizophrenia who had at least two admissions and whose second hospital admission lasted at least 30 days. These selection criteria resulted in a cohort of 458 chronically ill schizophrenic patients treated with conventional antipsychotics. The patients’ hospital admissions were followed for up to 2 years.
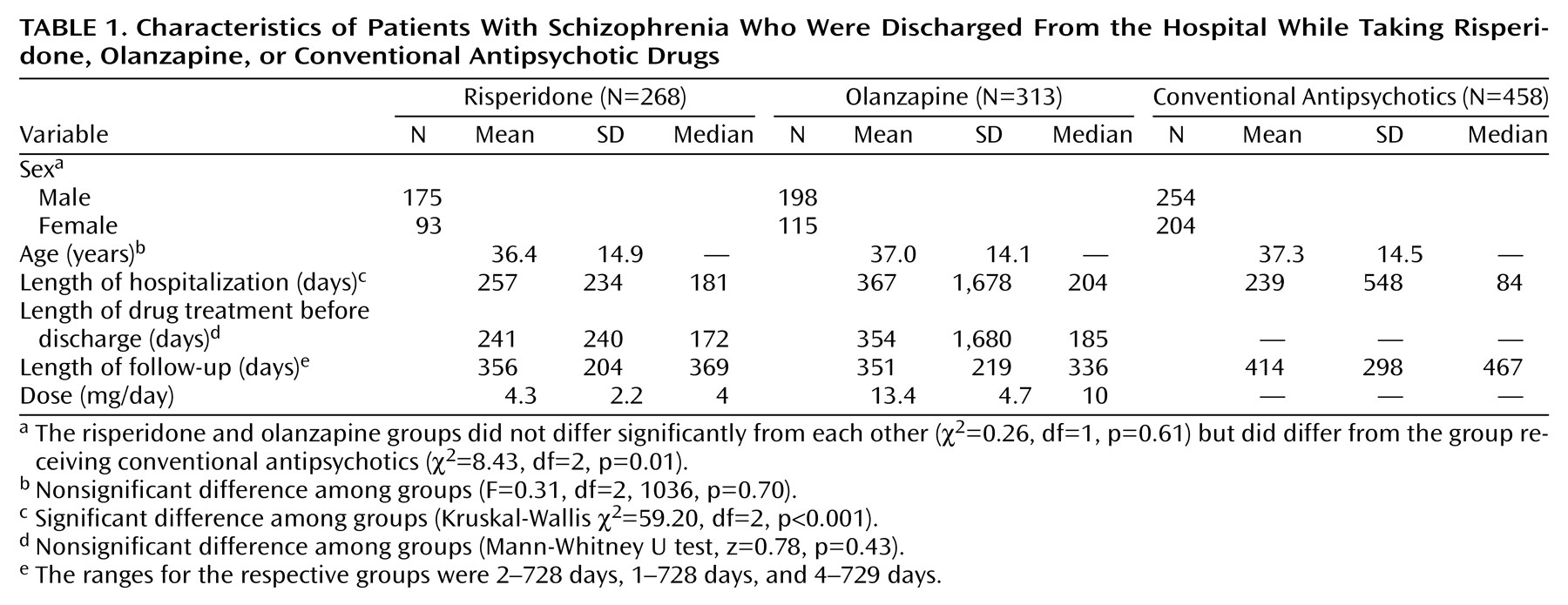
Characteristics of the three groups are listed in
Table 1, which shows that the group treated with conventional antipsychotics contained a higher proportion of women and had a shorter length of hospital stay and that the groups were approximately the same age. The mean lengths of follow-up time for the patients receiving risperidone and olanzapine were similar, and the follow-up was longer for the group receiving conventional antipsychotics, since more of them had a complete 2-year follow-up. In all but six cases we were able to verify that the patients treated with conventional agents were not included in the groups receiving novel antipsychotics.
Rehospitalization was defined as admission to any inpatient psychiatric facility. The risk factors for readmission that we examined were gender, age, time spent in the hospital before discharge, and for the two groups receiving novel antipsychotics, dose. All of the patients included in the study had a diagnosis of schizophrenia.
To measure time to readmission we used survival analysis, which takes into account differences in length of follow-up time. Survival curves were estimated by the product-limit (Kaplan-Meier) formula. The significance of differences among the three groups was measured by the Mantel-Cox chi-square test. The Cox proportional hazards regression model was used to analyze continuous covariates and those thought to affect time to readmission, such as age, dose, and sex. Standard chi-square tests, F tests, and nonparametric tests were used to compare demographic variables. All tests were two-tailed, and significance was defined as an alpha less than 0.05.
Results
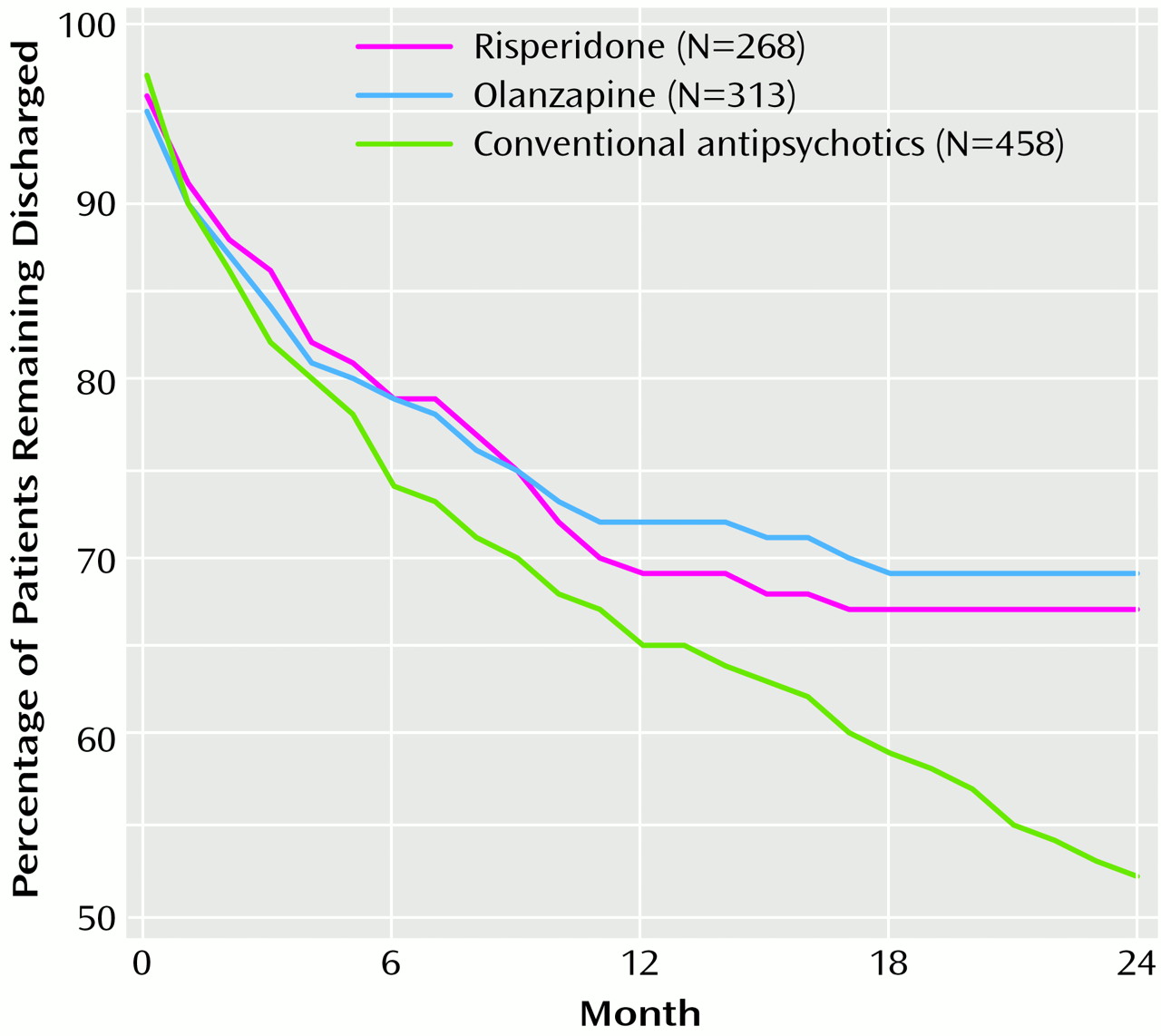
Figure 1 presents the time to readmission for each of the three groups. The cumulative percentages of patients who remained in the community were similar over the first 6 months (risperidone, 79%; olanzapine, 79%; conventional antipsychotics, 74%) and 12 months (risperidone, 69%; olanzapine, 72%; conventional antipsychotics, 65%). However, during the second year a clear advantage of the novel antipsychotics was evident. Whereas 67% of the risperidone group and 69% of the olanzapine group remained out of the hospital, this was true for only 52% of the group taking conventional antipsychotics (log rank=8.07, df=2, p=0.02). After we controlled for gender, which was associated with rehospitalization (men versus women: odds ratio=1.35, confidence interval=1.07–1.69, χ
2=6.69, df=1, p<0.01), and age, which was not (χ
2=0.13, df=1, p=0.72), the patients receiving risperidone and olanzapine, grouped together, were about one-quarter less likely (odds ratio=0.72, confidence interval=0.58–0.89, χ
2=9.25, df=1, p<0.002) to be readmitted than those getting conventional antipsychotics, according to the results of a Cox regression model. Additional Cox regression models were done for each drug individually to allow examination of the association of dose and rehospitalization. No relationship was found between dose and rehospitalization (χ
2<0.003, df=1, p<0.70).
Discussion
To our knowledge, this is the first nationwide study to compare patients taking risperidone, olanzapine, and conventional antipsychotics with respect to rehospitalization rates. The results of this study demonstrate that both risperidone and olanzapine are associated with rehospitalization rates lower than those for conventional antipsychotics. With regard to risperidone, Conley et al.
(6) reported that at 12 months 83% of patients had not been readmitted, and at 24 months the percentage was 66%, whereas we found 69% at 12 months and 67% at 24 months. Thus, whereas at 12 months the patients in the Conley et al. study did somewhat better than the patients we studied, by 24 months the rehospitalization rates in the two studies were almost identical.
Our rehospitalization rate for the group receiving conventional antipsychotics is in keeping with the rates reported by Hogarty
(7), which probably represent the rates of rehospitalization that would be expected with unselected conventional therapy and usual supportive care. Specifically, Hogarty
(7) reported that at 12 months 63% of patients had not been readmitted and at 24 months the rate was 45%, whereas we found 65% at 12 months and 52% at 24 months. Because the rate of rehospitalization for patients taking conventional antipsychotics increases considerably during the second year, it takes 2 years to clearly see the advantages of the novel antipsychotics.
The limitations of this study, similar to those in the study by Conley et al.
(6), are that the patients were not randomly assigned to the drug treatments and that no data were available on medication use after discharge. Thus, it is possible that the patients did not take their prescribed drugs for the entire follow-up period, and hence the results should be interpreted with caution. Another limitation is that since clozapine was not listed in the registry, some of the patients in the “conventional antipsychotic” group may have been given clozapine. To control for this possible bias, we compared this group to an additional group of patients (N=551) selected from the same national psychiatric registry but treated before the introduction of clozapine, or any other atypical antipsychotic, in April 1993. These patients were selected by using the same selection criteria, i.e., the eligibility criteria for treatment with novel antipsychotics. The two groups, one known to be taking only conventional antipsychotics, and a second that could have been “contaminated” by clozapine, had almost identical readmission rates at 6 months (74% versus 77%), 12 months (65% versus 64%), and 24 months (52% versus 49%). This suggests that the contamination is negligible and that it does not appear to affect the results of the study.
Another limitation is that, despite our use of criteria to make the patients receiving conventional antipsychotics as similar as possible to the patients taking the novel medications, the group taking conventional antipsychotics had a shorter mean length of hospital stay. This may have biased results in either direction. The shorter lengths of stay may mean that the patients taking conventional drugs, although matched to the patients treated with novel drugs in terms of chronicity and resistance to treatment, were in fact less ill than the patients receiving novel antipsychotics, and thus the real differences in rehospitalization rates between conventional and novel neuroleptics were probably greater than what we found. On the other hand, it is possible that some of the patients in the group treated with conventional antipsychotics would not have been rehospitalized if they had stayed in the hospital longer. In an additional analysis, not presented here, we used a more stringent length-of-stay inclusion criterion for the conventional antipsychotic group—60 days, as opposed to 30—and found almost identical results. Further evidence for the lack of bias introduced by length of stay is that it was not related to risk of relapse in the Cox regression model. Future studies should examine the occurrence of side effects and the criteria for response in relation to early readmission.
The results of the current study are important in informing the debate over the cost-effectiveness of the new antipsychotic agents, which are more costly than the conventional agents. The results suggest that the new drugs lower the risk for rehospitalization over 2 years by about one-third and thereby reduce the individual and societal costs of psychiatric hospitalization. Longer-term comparative follow-up studies evaluating readmission rates are needed for comparison of the second-generation antipsychotics to conventional antipsychotics.