Sundowning, or sundown syndrome, is a poorly defined entity. Some investigators even question its existence, pointing out that some patients are more “agitated” in the morning, some more in the afternoon and evening, and some equally agitated throughout the whole day
(2,
3). Sundowning has been also ascribed to environmental influences, such as change of shift
(4), or to attempts of confused individuals to leave their units because they “want to go home from work.” There is also no agreement about the time of the day when increased symptoms must occur to be considered sundowning. Some investigators include only increased symptoms during the afternoon and early evening, while others also include behavioral symptoms throughout the night
(5). In this study we defined sundowning as “the appearance or exacerbation of behavioral disturbances associated with the afternoon and/or evening hours”
(1).
Several physiological processes that regulate body functions and behavior are influenced by circadian rhythms. Circadian rhythmicity, regulated by the suprachiasmatic nucleus, is observed in body temperature, heart rate, secretion of several hormones, red blood cell production, and other physiological characteristics
(6). Alzheimer’s disease leads to pathological changes in the suprachiasmatic nucleus
(7,
8) and disruption of circadian rhythms. Compared with healthy elderly individuals, individuals with severe Alzheimer’s disease
(9) had a greater percentage of their total daily motor activity during the night, lower amplitude of 1 cycle/day motor activity rhythm, and later acrophases (time of peak values) of both motor activity and body temperature rhythms. A subgroup of patients with large mean time differences between the acrophases of their activity and temperature cycles had lower temperature amplitudes and greater activity during the night than patients whose motor and temperature rhythms peaked together. Therefore, it is possible that behavioral symptoms that exhibit diurnal variation, such as sundowning, are related to the disruption of circadian rhythms.
In the present study we investigated the relationship between sundowning and characteristics of circadian rhythms in a group of ambulatory patients with Alzheimer’s disease. In addition, characteristics of their circadian rhythms were compared with those of age-matched comparison subjects.
Method
Subjects
All of the subjects with Alzheimer’s disease were inpatients on the dementia study unit at a Department of Veterans Affairs hospital. The study group consisted of 25 male patients with a mean age of 71.0 years (range=60–88) and a mean duration of illness of 11 years (range=5–25). All met the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
(10) for probable Alzheimer’s disease and the DSM-III-R criteria for dementia of the Alzheimer type, and all of them had moderate or severe cognitive impairment. The patients had no prior history of any psychiatric or neurological disorder in addition to Alzheimer’s disease. They were free of acute medical illness and were not febrile during the periods in which they were studied. The most commonly administered centrally acting medications were neuroleptics (19 subjects), diphenhydramine (eight subjects), short-acting benzodiazepines (five subjects), antidepressants (three subjects), phenytoin (three subjects), and chloral hydrate (two subjects). None had taken any antipyretic or sedative medication for at least 24 hours before being studied.
The comparison subjects were nine male healthy volunteers with a mean age of 73.4 years (range=67–83), studied immediately after admission to a similar clinical research unit. They had no cognitive impairment or history of psychiatric or neurologic disorders and were free of medications.
Procedures
After institutional review and approval of the project, written statements of informed consent were obtained from the comparison subjects and from the next of kin of the demented subjects after a full explanation of the study procedures. The patients and healthy individuals were studied concurrently to control for potential seasonal effects. Mealtimes were fixed and similar for the patients and the comparison group. The healthy individuals were housed on a research ward and allowed to follow their usual patterns of activity, rest, and sleep. The patients had twice-daily nursing care at fixed times, and all were exposed to the same regular pattern of lights on (6:00 a.m.) and off (10:00 p.m.).
A portable AM-16 activity monitor with solid-state memory (Ambulatory Monitoring, Ardsley, N.Y.), worn in a pocket of a vest and positioned at waist level, was used to monitor locomotor activity. The monitor is sensitive to accelerations of 0.1 g (one activity count) by the mechanical action of a piezoelectric bender and counterweight, and it was programmed to accumulate activity counts in 5-minute epochs over 72 hours by using the monitor’s zero-crossing mode. This mode measures the oscillation of the bender and allows for more precise quantification of extreme movements than is possible with the monitor’s threshold mode. Core body temperature was measured with a temperature-sensitive thermistor probe (YSI series 400, Yellow Springs, Ohio), sensitive to 0.1°C and inserted 10 cm into the rectum. The probe was connected to an ambulatory recorder (Mini-Logger, Mini-Mitter Co., Sunriver, Ore.). Temperatures were sampled every 6 minutes for 72 hours, concurrently with the activity monitoring.
Severity of dementia was measured by the Mini-Mental State
(11), by the Language Assessment Scale
(12), which is based on the Boston Naming Test
(13), and by the Bedford Alzheimer Nursing Scale—Severity
(14). The Mini-Mental State and Language Assessment Scale were administered by a research assistant, and the Bedford Alzheimer Nursing Scale—Severity score was determined by interviews of staff members with direct knowledge of the patients during the morning, afternoon, and evening hours over the previous 4 weeks. The same staff members also rated each patient for the presence of sundowning and sleep-wake disturbances, and the final rating was a consensus obtained by interviewing staff on all shifts. The staff was well educated in concepts of behavioral symptoms of dementia and had participated in several previous studies of sundowning
(1,
15). The staff members were told that the definition of sundowning is “the appearance or exacerbation of behavioral disturbances associated with the afternoon and/or evening hours” (i.e., from 3:00 to 11:00 p.m.). The choices for sundowning were “never,” “rarely,” “sometimes,” and “usually.” The choices for sleep-wake disturbances were “usually has a regular sleep-wake cycle,” “sometimes has an irregular sleep-wake cycle,” “frequently exhibits an irregular sleep-wake cycle,” and “severely disrupted sleep-wake cycle.” The patients’ current ages and ages at the onset of dementia symptoms were obtained by chart reviews.
Data Analysis
The data were analyzed by the same procedures as those used in our previous study
(9). The activity data were first analyzed for measures of gross motor activity, including mean nocturnal and diurnal activity levels and percentage of nocturnal activity. For the purposes of this study and for consistency with our previous study
(9), diurnal activity was defined as that occurring between 6:00 a.m. and 10:00 p.m.; nocturnal activity occurred from 12:00 a.m. to 6:00 a.m. “Percentage of nocturnal activity” refers to the percentage of total daily activity occurring during the nocturnal period. In addition, several assessments of the circadian rhythm of locomotor activity were made: interdaily stability—a nonparametric, periodogram-based measure of day-to-day stability of the circadian rhythm of activity, where a higher value indicates greater stability
(16)—and a cosinor analysis of the circadian rhythm yielding the mesor—central point of the fitted, circular (cosinor) function—amplitude (height of the fitted cosine curve), relative amplitude (amplitude/mesor), and acrophase (clock time of peak value)
(17). In addition, the correlation between the cosinor model and the raw data was calculated to determine the goodness of fit. Core body temperature was assessed by using cosinor analysis and spectral analysis (1 cycle/day variance)
(17).
Differences between the mean values for the circadian rhythm measures and test scores of the patients and comparison group were analyzed for statistical significance by unpaired t tests with separate variances because of the difference in group sizes. The normality assumption was tested by the Kolmogorov-Smirnov test, and the equal-variance assumption was tested by the Levene median test. All assumptions were valid at a criterion level of p>0.05.
The significance of differences among patients grouped according to the degree of sundowning was first analyzed by an analysis of variance (ANOVA). For this analysis, patients exhibiting sundowning “never” and “rarely” were combined into one group because the differences in circadian variables between those two groups were only those expected by chance. Only two patients had sleep-wake disturbances more than “sometimes has an irregular sleep-wake cycle.” Therefore, we analyzed the data for differences between the patients who were rated as “usually has a regular sleep-wake cycle” and the patients who had some abnormalities of the sleep-wake cycle. The Fisher least-significant-difference multiple comparison test was used to determine the significance of differences between groups. The Pearson correlation coefficient was used to assess the relationship between reported sundowning and disturbances of the sleep-wake cycle.
Results
The patients with Alzheimer’s disease included in this study had moderate to severe dementia. Their mean Mini-Mental State score was 0.7 (range=0–11), their mean score on the Language Assessment Scale was 7.3 (range=4–15, where 20=normal and 4=most impaired), and their mean score on the Bedford Alzheimer Nursing Scale—Severity was 16.2 (range=11–22, where 7=normal and 28=most impaired). Nine patients were able to walk independently at all times, four required occasional assistance, and 12 walked only with assistance.
As shown in
Table 1, the patients with Alzheimer’s disease had less diurnal motor activity and a higher percentage of nocturnal activity than the comparison group. Their motor activity also showed less interdaily stability and had a later acrophase. The measurements of core body temperature showed that the patients with Alzheimer’s disease had a higher temperature mesor, greater amplitude of the temperature curve, and a later acrophase than did the comparison group
Table 1 also displays data from our previous observations of nonambulatory patients
(9), and it can be seen that the values for most variables deviate from normal in the same direction in both groups with Alzheimer’s disease but that the degrees of deviation are smaller for the ambulatory patients. This pattern suggests that the circadian abnormalities progress together with cognitive and functional deterioration. The only abnormalities that were not found in our previous study were the high temperature mesor and amplitude.
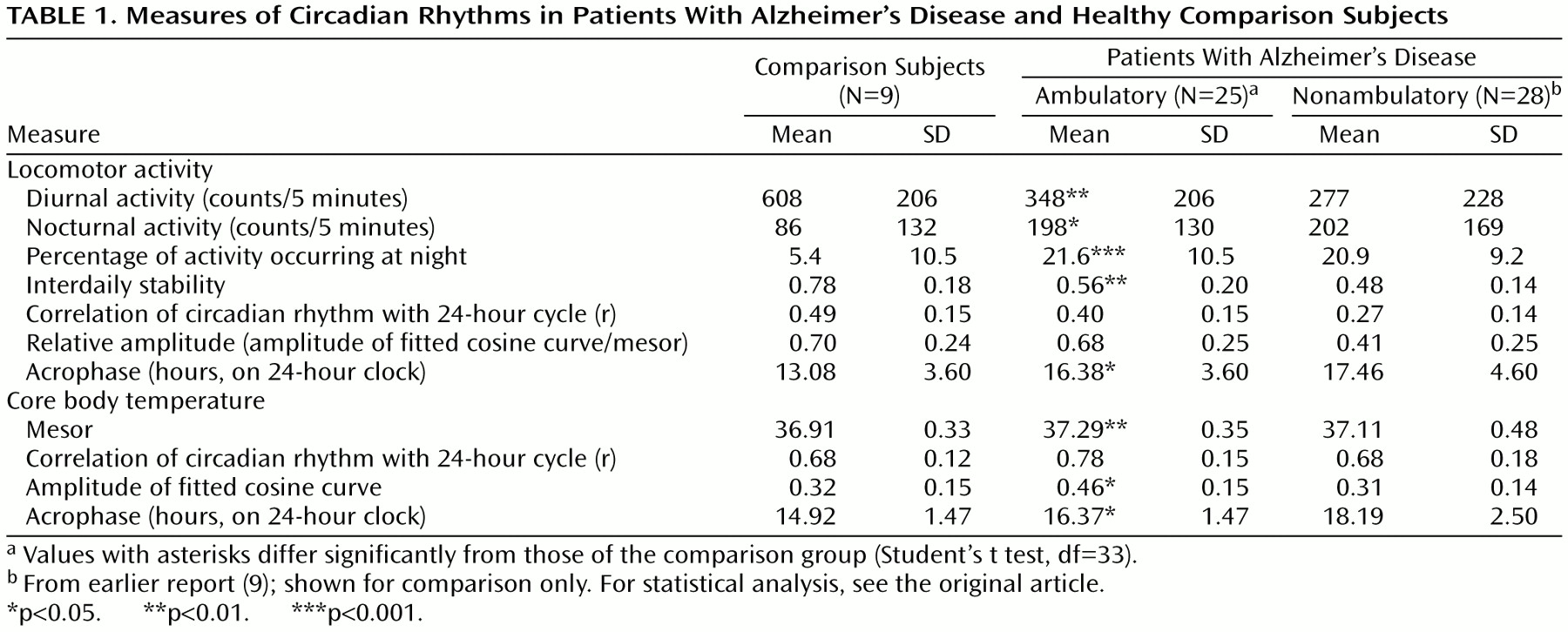
The staff reported that 14 of the patients with Alzheimer’s disease sundowned never or rarely, eight patients sometimes, and three patients usually. The mean ages, ages at onset of dementia, and severity of dementia were similar in the three groups. Although the patients who exhibited sundowning had higher levels of total activity, the differences were not significant (
Table 2). Some patients who exhibited sundowning had increased locomotor activity in the evening, but others had similar activity throughout the day (
Figure 1).
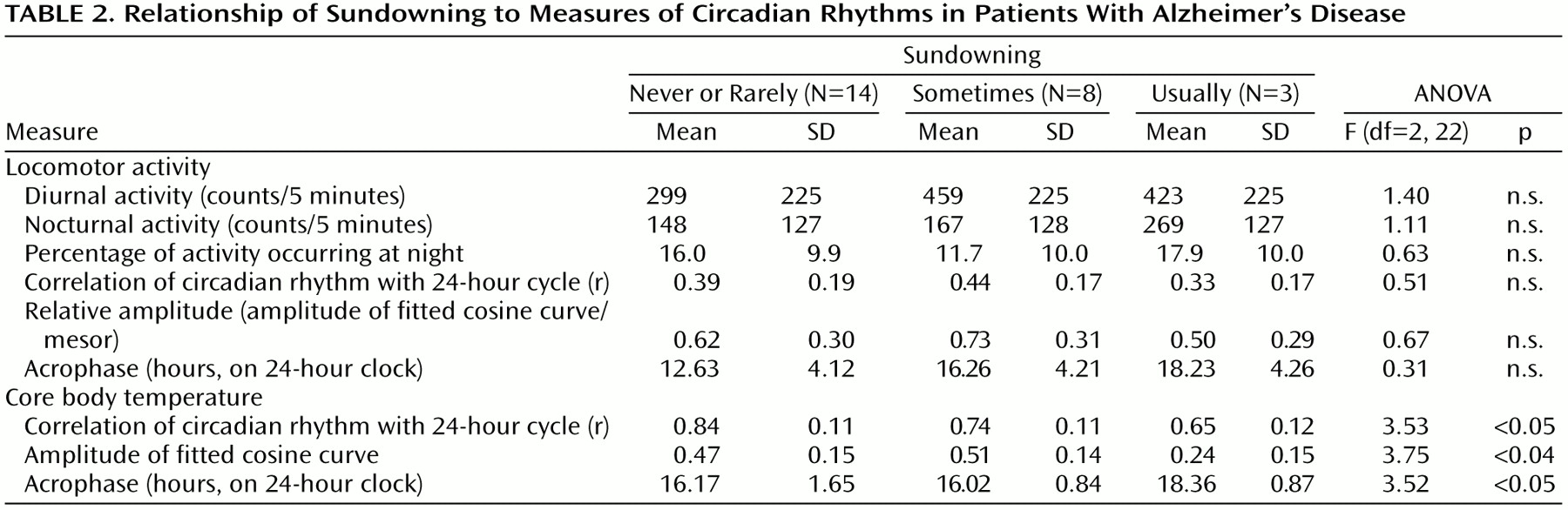
According to ANOVAs, the sundowning status was significantly related to several circadian characteristics (
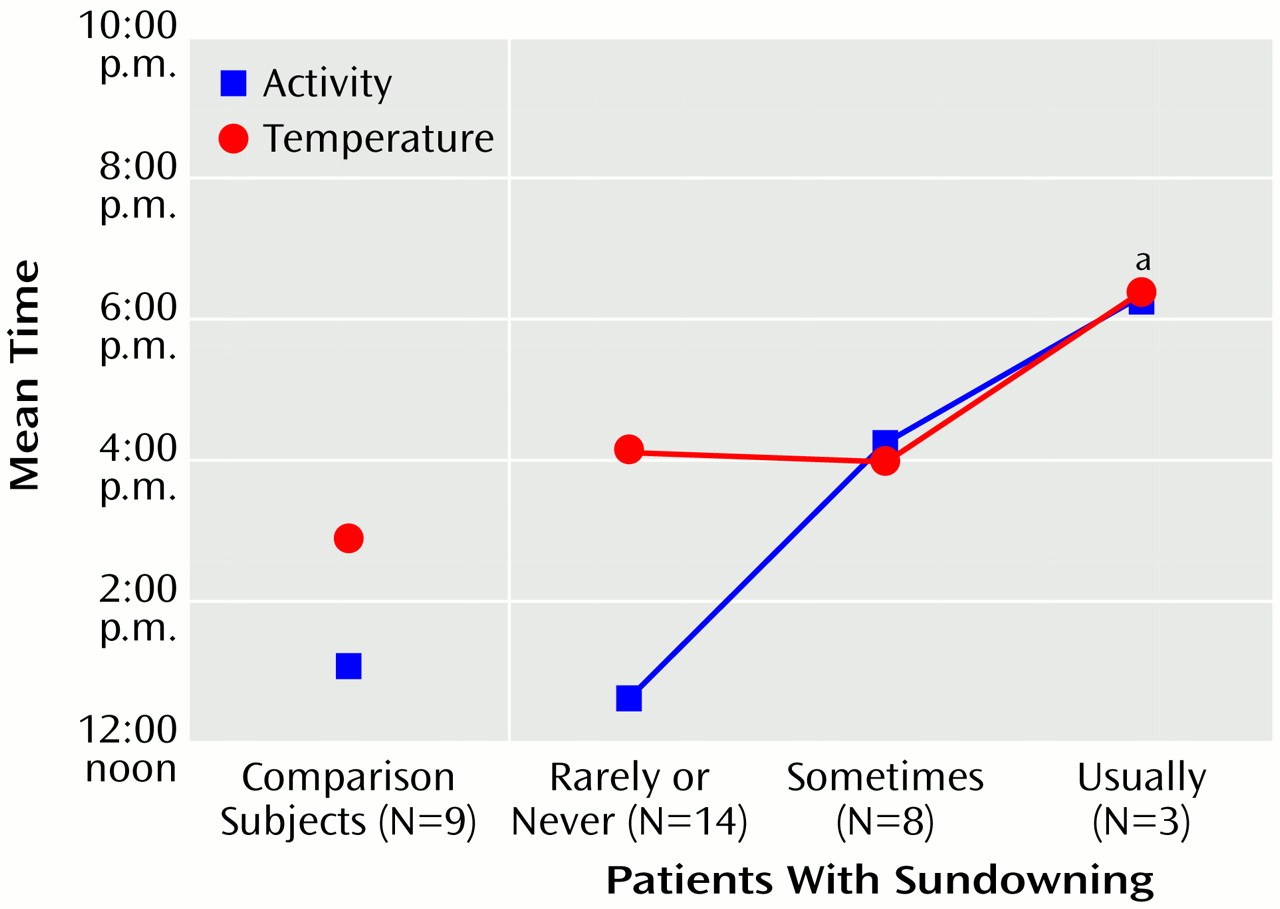
Figure 2). The acrophase of motor activity occurred later in the patients who exhibited sundowning than in both the patients who did not exhibit sundowning and the comparison group (F=3.46, df=2, 22, p<0.05) (
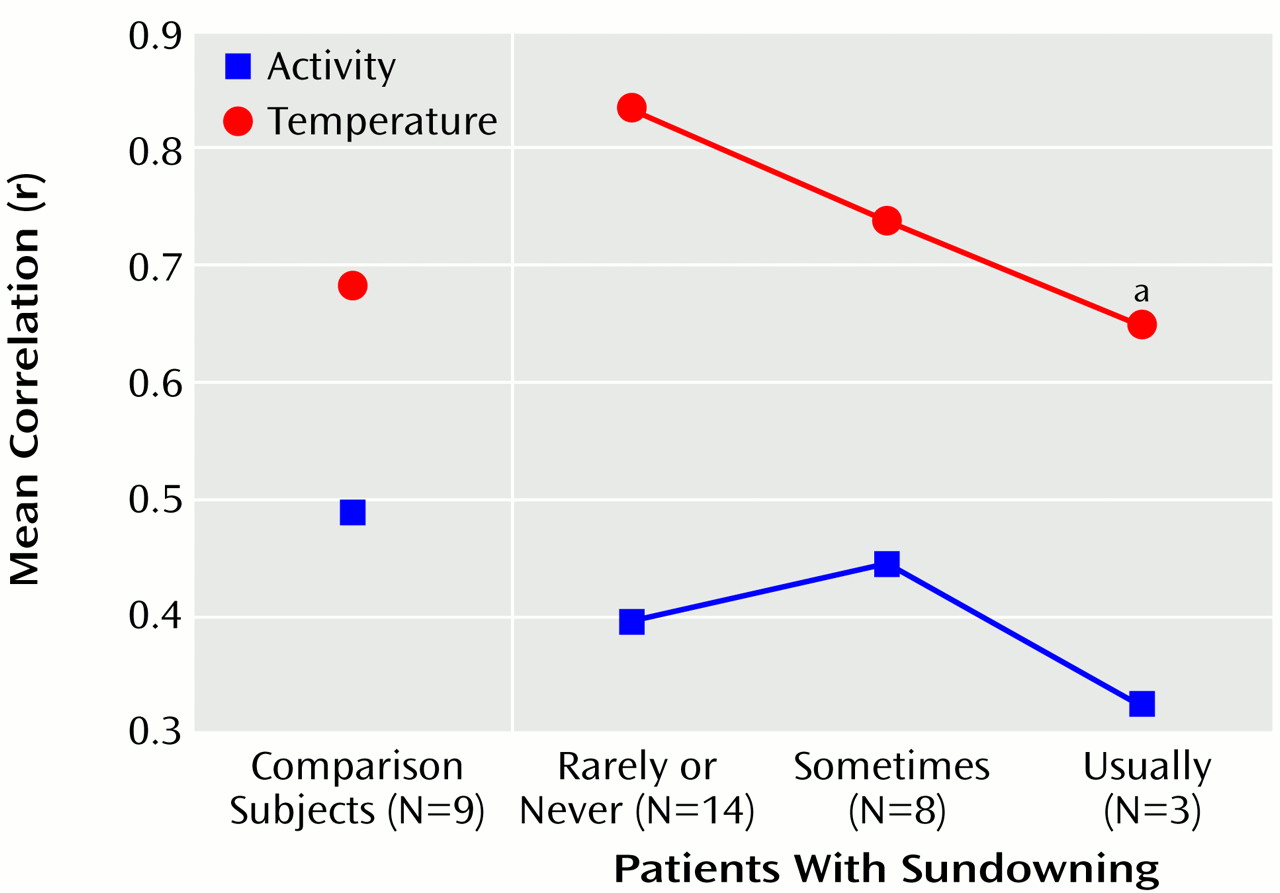
Figure 3). The acrophase of core body temperature occurred later in individuals who usually exhibited sundowning than in individuals who exhibited sundowning less often (F=3.52, df=2, 22, p<0.05). The goodness of fit of the temperature data with a 24-hour cycle decreased with increasing severity of sundowning (F=3.53, df=2, 22, p<0.05) (
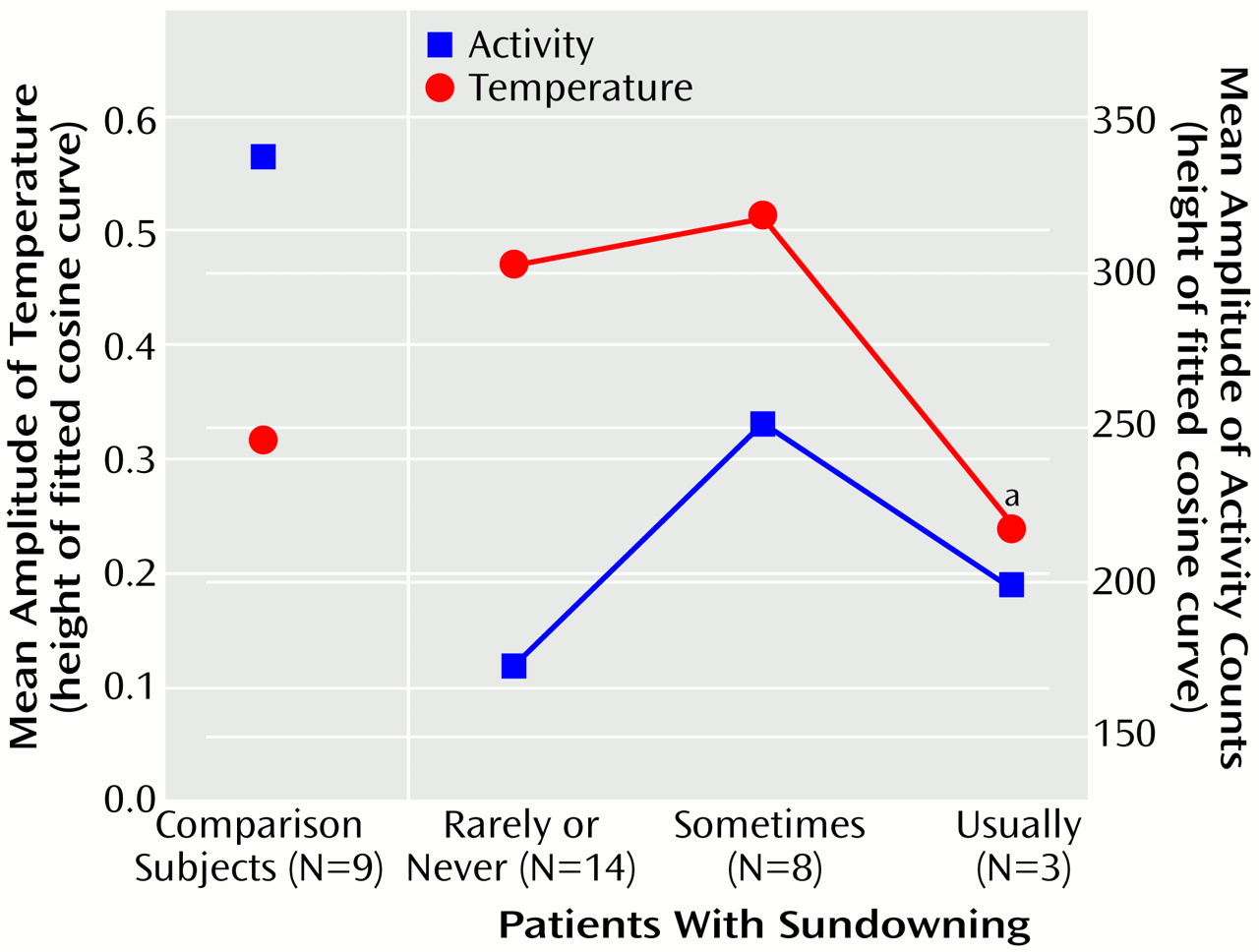
Figure 4). The amplitude of the circadian temperature cycle was lower in the patients who usually exhibited sundowning than in those who exhibited sundowning less often (F=3.75, df=2, 22, p<0.05) (
Figure 5).
The sundowning status was not related to disturbances of the sleep-wake cycle. According to the staff ratings, 15 subjects had regular sleep-wake cycles, eight patients sometimes had irregular sleep-wake cycles, and one patient each had “frequently irregular” and “severely disrupted” sleep-wake cycles. There was no significant correlation between the staff ratings of sleep-wake disturbance and sundowning (Pearson r=0.26, df=24). Actually, the three patients who exhibited the most sundowning had either regular sleep-wake cycles (two patients) or an only sometimes irregular sleep-wake cycle (one patient). The three sundowning levels did not differ significantly in percentage of motor activity occurring during the night (
Table 2), but the relative activity amplitudes were significantly different in patients who had and did not have disturbed sleep-wake cycles (F=7.74, df=2, 22, p=0.01). Patients with normal sleep had a higher mean relative activity amplitude (mean=0.45, SD=0.03) than patients with disturbed sleep (mean=0.29, SD=0.04) (Fisher least significant difference, p<0.05), while the comparison subjects had an intermediate value (mean=0.39, SD=0.03). The other significant difference in circadian rhythm characteristics between the patients who had and did not have disturbed sleep-wake cycles was in the degree of fit of the motor activity variation to a 2 cycle/day model (mean=0.29, SD=0.08, versus mean=0.45, SD=0.17) (F=7.74, df=2, 23, p=0.01).
Discussion
The disturbances of circadian rhythms in the ambulatory patients with Alzheimer’s disease included in this study were similar but less pronounced than the disturbances detected in the more severely demented nonambulatory patients studied previously
(9,
18). The only exceptions were the higher mesor and 1 cycle/day amplitude of the circadian temperature curve. The temperature mesor was also higher in individuals with Alzheimer’s disease than in the comparison group in our previous study, but the difference was not statistically significant. The reason for the difference between studies is not clear. None of the patients in this study had evidence of an intercurrent infection. It is possible that Alzheimer’s disease affects hypothalamic function and thus interferes with normal regulation of core body temperature. Neurofibrillary tangles are present in the hypothalamus of people with Alzheimer’s disease
(19), and neurochemical abnormalities include lower choline acetyltransferase activity and serotonin concentration
(20). The higher amplitude of the circadian cycle of body temperature observed in this study may be also related to this hypothalamic dysfunction. Alternatively, the higher mesor of body temperature could be due to low melatonin secretion, which has been described in patients with Alzheimer’s disease
(21).
Earlier studies
(22,
23) suggested an inverted-U relationship between impairments of the circadian rhythm and severity of dementia. The results of this study combined with the results of our previous study
(9) demonstrate that impairment of the activity and temperature rhythms follows a gradual progression from mild to severe during the course of the illness. Final clarification of the relationship between circadian rhythm changes and dementia progression will require longitudinal study of the changes in circadian rhythms induced by Alzheimer’s disease.
Sundowning, defined in this study as “the appearance or exacerbation of behavioral disturbances associated with the afternoon and/or evening hours”
(1), is common in patients with moderate and severe Alzheimer’s disease. In this study, sundowning was present in 11 (44%) of our 25 patients. This prevalence is higher than the reported prevalence in a general nursing home population (13%)
(24) but lower than the reported prevalence in patients with Alzheimer’s disease living at home (66%)
(25). The study of patients living at home, however, included behavioral disturbances both during the afternoon and during the night.
The results of this study indicate that circadian rhythm disturbances are present in patients who exhibit sundowning as we have defined it. The most significant finding was later acrophase of core body temperature. This delay may indicate a decreased ability of the suprachiasmatic nucleus to synchronize endogenous rhythms with environmental cues. Other indications that circadian rhythms in sundowning individuals are impaired include reduced goodness of fit of the circadian rhythm of core body temperature to a 24-hour cycle and a lower amplitude of the 1 cycle/day temperature curve. These impairments of circadian rhythms may be responsible for the behavioral symptoms that are exhibited during episodes of sundowning, but it is also possible that sundowning induces alterations in circadian rhythmicity.
The results of this study have to be considered preliminary because of several limitations. First, the patients with Alzheimer’s disease had been hospitalized for extended periods, while the comparison individuals were studied soon after admission to a research ward. Hospital routines and environment may alter circadian rhythmicity and, therefore, could be responsible for some of the differences seen in this study. However, we recently reported
(26) that the abnormalities of circadian rhythms in patients institutionalized for long periods who had pathologically confirmed Alzheimer’s disease differed from the circadian rhythm abnormalities of patients with frontotemporal dementias. This finding does not support a systematic, nonspecific impact of the environment on the expression of rhythmicity in this population. Second, the removal of sedative medications from the daily regimens of some of the patients could have had additional effects on their state of agitation. Measurement of behavioral indices, including sundowning measures, following a 2-week or longer washout of sedating medications followed by circadian measurements could shed further light on these phenomena. Third, we measured several circadian characteristics, and their analysis required multiple comparisons. Thus, it is possible that some differences are due to type I errors. However, the consistency of the results between this and the previous study
(9) minimizes the possibility that the differences are due to chance.
The comparison group had a lower temperature amplitude and goodness of fit to a cosinor model than the never- and sometimes-sundowning patients with Alzheimer’s disease. This can be best explained by reference to
Figure 2. The temperature rhythm in the comparison group, while having robust circadian characteristics, was often masked by the subjects’ volitional activity, which varied throughout the day. Therefore, the overall amplitude and goodness of fit are lower than in most of the patients with Alzheimer’s disease, whose activity patterns were more regular. It remains to be seen whether this pattern would still be true if all groups were studied under constant routine conditions
(27), which would eliminate masking of the temperature rhythm by sleep and other behaviors. Even though the measures of goodness of fit and amplitude in the comparison group and the usually sundowning group were similar, the patterns of their temperature and activity were grossly divergent. The usually sundowning patients appeared to have a chaotic temperature rhythm that had little relationship to the activity record.
Our data indicate that increased motor activity is not a necessary concomitant of sundowning. Previous studies used instruments that combined all behavioral symptoms in one score, such as the Cohen-Mansfield Agitation Inventory
(2) and the Confusion Inventory
(24). Analysis of data obtained through the Confusion Inventory did not show a clear pattern of sundowning behavior and included restlessness, escape behaviors, expression of feelings, talking and other verbalizations, and the appearance of searching as aspects of sundowning
(24). Other instruments rely on staff reports regarding the presence of seven disruptive behaviors (combativeness, agitation or purposeless movement, wandering, prolonged incoherent vocalization, hallucinations, confusion, and disorientation)
(25) or observation of five behaviors (carphologic [restless], restraint removal, searching, tapping or banging, and vocalization)
(28). It is most likely that behaviors that are exhibited during the sundowning episode differ from patient to patient and may represent increased intensity of behaviors that the patients exhibit at other times as well.
The acrophase of core body temperature seen in the normal elderly subjects in this study is earlier than that cited in some other studies
(27,
29). There are several possible reasons for our different results. Core body temperature in our subjects was evaluated without the benefit of a constant routine. The impact of influences such as sleep and activity, which can mask the circadian rhythm and which a constant routine eliminates, can have profound effects on the expression of the rhythm of core body temperature and could account for the relative phase advance observed in our normal elderly subjects. Studies of circadian rhythm of core body temperature in normal elderly subjects have shown high variability, often 2–3 hours, in the time of the temperature nadir, a precise circadian phase marker, and this could also account for our different measurement of acrophase. Last, it is also possible these other groups of normal elderly subjects may have contained some with early Alzheimer’s disease or mild cognitive impairment that went undetected by the screening procedures used in those studies. Further studies with more rigorous control of subjects’ masking behaviors and with patients in earlier stages of Alzheimer’s disease could help in determining the cause of this discrepancy.
In our patient group, sundowning and sleep disturbances were two unrelated symptoms of dementia. It is possible that a phase delay of circadian rhythms is more closely related to sundowning, while other consequences of Alzheimer’s disease, such as decreased 2 cycle/day rhythm or loss of serotonin and other neurotransmitters, are responsible for sleep disturbances. There are also many other possible causes of sleep disturbances, such as sleep-related motor disorders and sleep-related respiratory disorders, which are more common in demented individuals
(30). The therapeutic goals for sleep disturbances are different from the goals in the treatment of sundowning. Treatment of sleep disturbances promotes undisturbed sleep, while management of sundowning strives to maintain patients’ safety and comfort without sedation and sleep. On the basis of the lack of association between sundowning and sleep-wake disturbances, their association with different circadian rhythm abnormalities, and the different objectives in treating them, we propose that the term “sundowning” be limited to behavioral disturbances during the afternoon and evening hours, when the patient is expected to be awake.