Attention deficit hyperactivity disorder (ADHD) is a childhood-onset psychiatric disorder whose cardinal symptoms are inattention, hyperactivity, and impulsivity. Although the details of ADHD’s etiology and pathophysiology are still being worked out, the available data implicate dysregulation of catecholaminergic systems
(1), and several lines of data implicate genes in the etiology of ADHD
(2). The biological relatives of children with ADHD are at greater than normal risk for ADHD, twin studies show ADHD to be highly heritable, and adoption studies suggest that biological, not adoptive, relationships mediate the familial transmission of the disorder. Although ADHD’s mechanism of inheritance is unknown, its high population prevalence (5% to 10%) and modest risk to first-degree relatives (about 15% to 20%) suggest that the mechanism of transmission is complex
(3).
Molecular genetic studies of ADHD have focussed on genes in catecholaminergic pathways because animal models, theoretical considerations, and the effectiveness of stimulant treatment implicate catecholaminergic dysfunction in the pathophysiology of the disorder
(1). The dopamine D
4 receptor gene (DRD4) was examined in many studies, after LaHoste et al.
(4) found that ADHD patients were more likely to carry the DRD4 7-repeat allele than were comparison subjects. Since that initial report, there have been several attempts to replicate this finding by using either case-control studies
(5–
11) or family-based association studies
(6,
7,
9–
20). Unfortunately, neither type of study has consistently confirmed the putative association between ADHD and the DRD4 7-repeat allele.
These ambiguous findings for ADHD and DRD4 are not unusual for psychiatric genetic studies
(3). Suarez et al.
(21) used statistical simulations to illustrate one cause of this problem. Their simulations were based on the assumption that, because psychiatric disorders are likely to be mediated by many genes acting in concert, each of these genes individually exerts only a small effect on the disorder. Although genes of such small effect should be difficult to detect, because many are involved, the power of an initial study to detect one gene from the set should be reasonably high. The simulations of Suarez et al. showed that a replication study would also have reasonable power to detect one gene from the set but that the probability that the same gene would be detected would be low. For the replication study to have sufficient power to detect the previously detected gene, it must use a much larger sample.
To deal with the ambiguities raised by inconsistent results among molecular genetic studies, Rice
(22) suggested that the statistical method of meta-analysis be used to reconcile conflicting findings. This method is used to examine whether the aggregate evidence across all available studies provides evidence of statistical significance. Thus, to examine the putative association between ADHD and the DRD4 7-repeat allele, we applied meta-analysis to all available case-control and family-based association studies.
Method
We identified all available studies of the association between ADHD and DRD4 by 1) searching journal abstracts available online through PubMed at the National Library of Medicine, 2) requesting ADHD-DRD4 association data from colleagues presenting such data at national meetings, and 3) querying members of the ADHD molecular genetics e-mail network (funded by NIMH) about the existence of other ADHD-DRD4 data sets that had been published or were being prepared for publication (these latter studies were used with permission of the authors). All studies identified were included.
All of the data we analyzed have been previously published with two exceptions. Data presented at a meeting by one of us
(20) are an expansion of those in a published report
(13). The data set of Castellanos, described in a conference report
(18), expands on a published work by Castellanos et al.
(5). The studies that provided case-control and family-based data are described in
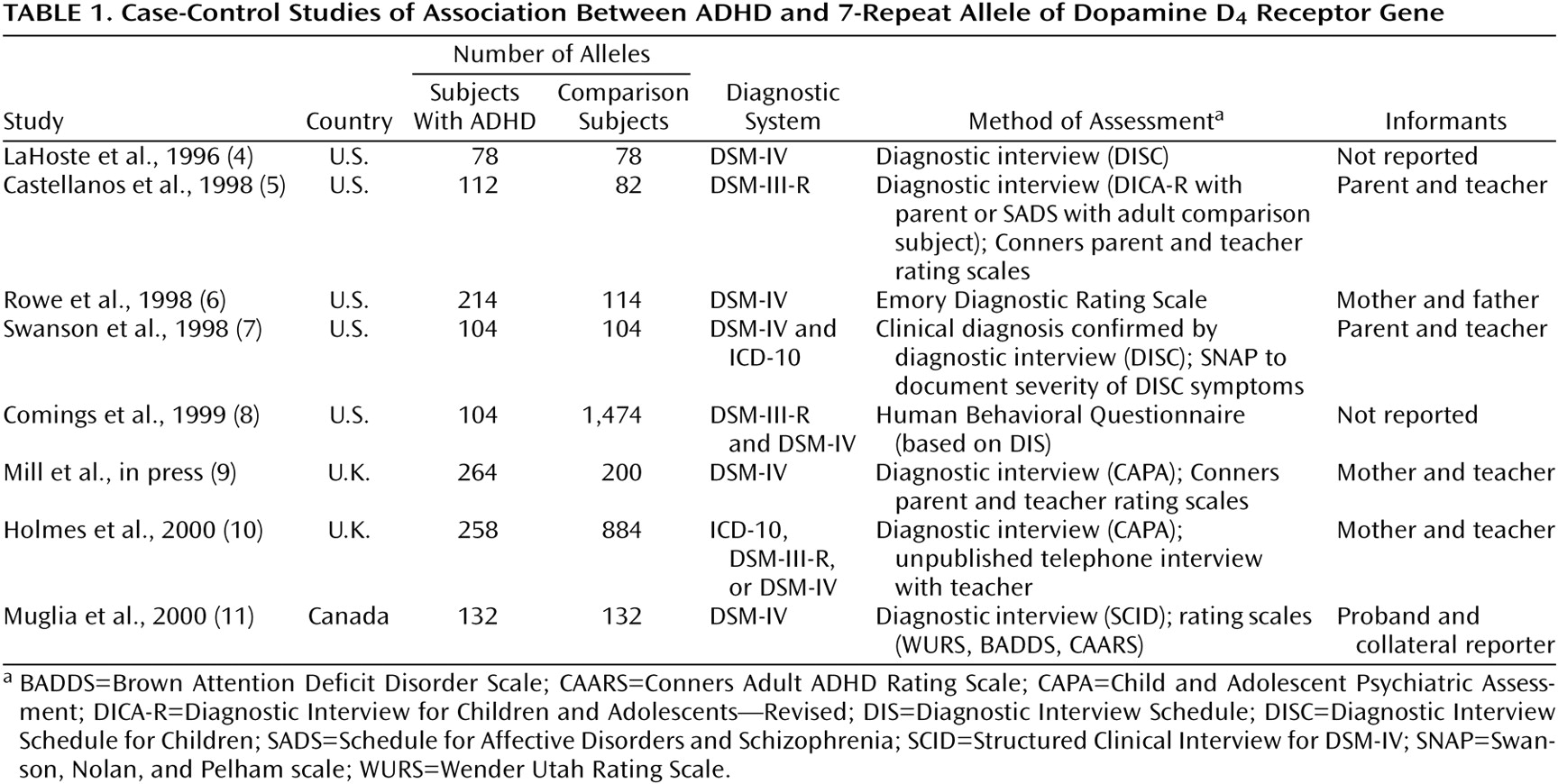
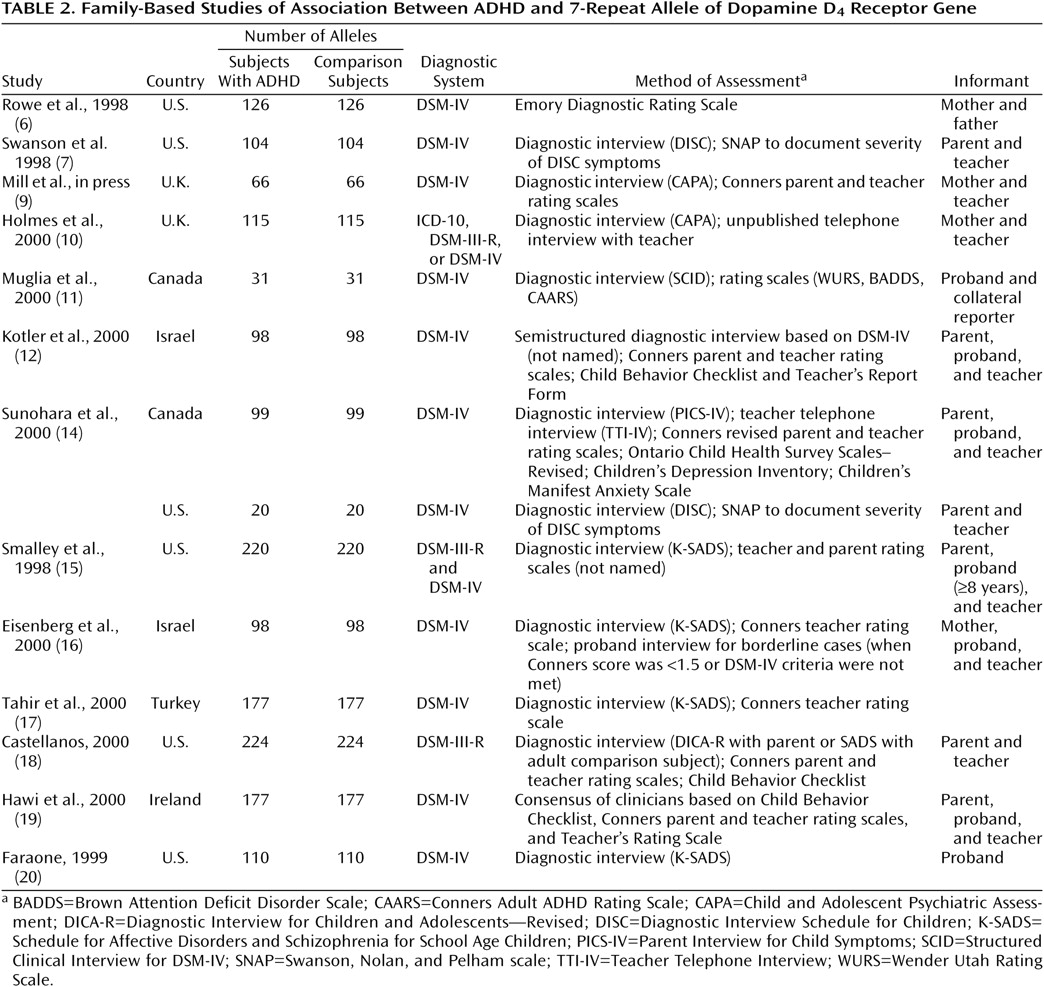
Table 1 and
Table 2, respectively. Studies that provided both types of data are described in both tables.
We performed two meta-analyses, one for the case-control studies and one for the family-based studies. For the case-control data, each study provided the two-by-two table classifying subjects by diagnosis (ADHD or not) and DRD4 7-repeat allele status (present or not). For the family-based data, each study provided the two-by-two haplotype-based haplotype relative risk table, which classifies parental alleles by type of allele (7-repeat or not) and transmission status (transmitted to the ADHD child or not)
(23). We summarized the strength of association in these two-by-two tables by using the odds ratio. For case-control studies, the odds ratio is an estimate of the relative risk, which indicates the odds of having the 7-repeat allele among individuals with ADHD in relation to the odds for individuals without ADHD. For family-based studies, the odds ratio is an estimate of the haplotype relative risk, the odds of transmission to individuals with ADHD of the 7-repeat allele in relation to other alleles. Knapp et al.
(24) showed that when there is no recombination between the marker and disease gene, haplotype relative risk is equal to relative risk. Otherwise, when marker and disease show a positive association, relative risk is equal to or greater than haplotype relative risk. We also computed the attributable fraction in the population, which indicates the fraction of ADHD cases in the population that can be attributed to the DRD4 7-repeat allele.
We used a random effects meta-analysis to analyze the odds ratios by using the method of Carlin
(25). To determine whether the results of the meta-analysis were unduly influenced by any one study, we recomputed the meta-analysis statistic after deleting each study one at a time. We assessed publication bias by using the method of Egger et al.
(26). This method is based on the fact that the precision of the odds ratio increases with larger study groups. The method regresses the standard normal deviate of the odds ratio (the odds ratio divided by its standard error) against the precision of the odds ratio (the inverse of its standard error). In the absence of bias, Egger et al. showed that the regression of the standard normal deviate on precision of the odds ratio should run through the origin (i.e., small study groups with low precision have large standard errors and therefore small standard normal deviates; large study groups have higher precision, smaller standard errors, and large standard normal deviates). The publication bias statistic of Egger et al. is the intercept of the regression, which will be significantly greater than zero in the presence of publication bias. For all analyses we used Stata 6.0
(27).
Results
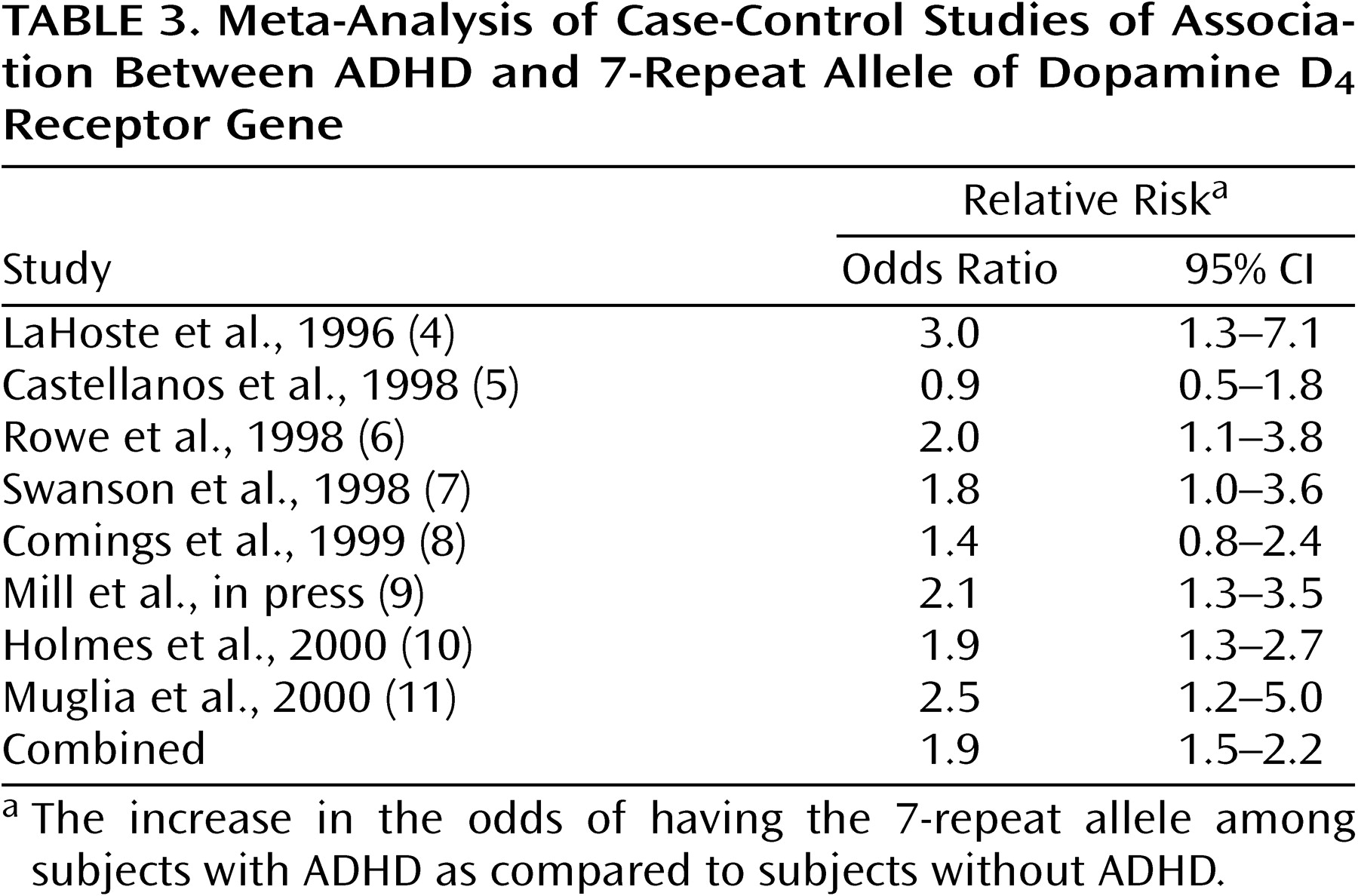
Table 3 gives the odds ratios and their 95% confidence intervals (CIs) for the eight case-control studies. Five of eight studies showed a significant association between ADHD and the DRD4 7-repeat allele, as indicated by a CI not including 1.0. The combined estimate was 1.9 and was statistically significant (z=5.2, p<0.001; 95% CI for odds ratio=1.5–2.2). There was no statistically significant evidence for heterogeneity of the odds ratio among the case-control studies (χ
2=6.5, df=6, p=0.42). The population attributable risk was 0.14.
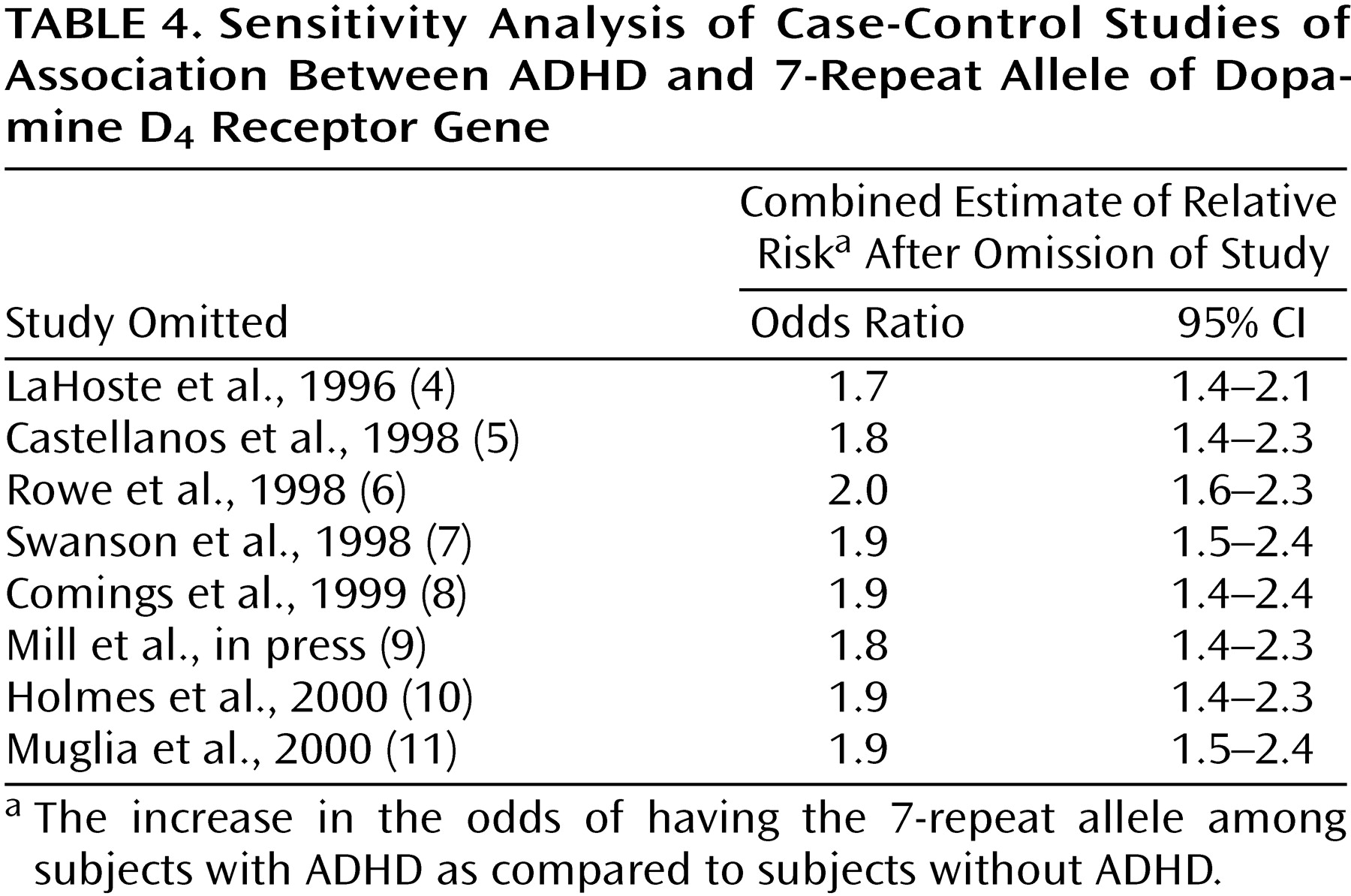
Table 4 presents a sensitivity analysis in which the combined estimate of the odds ratio was computed after omission of one study at a time. This analysis shows whether the significance of the combined estimate can be attributed to a single study.
Table 4 shows that the estimates of the combined odds ratio range from 1.7 to 2.0, suggesting that no one study is heavily influencing the combined estimate. Moreover, the CIs in
Table 4 show that the combined odds ratio retains statistical significance regardless of which study is deleted.
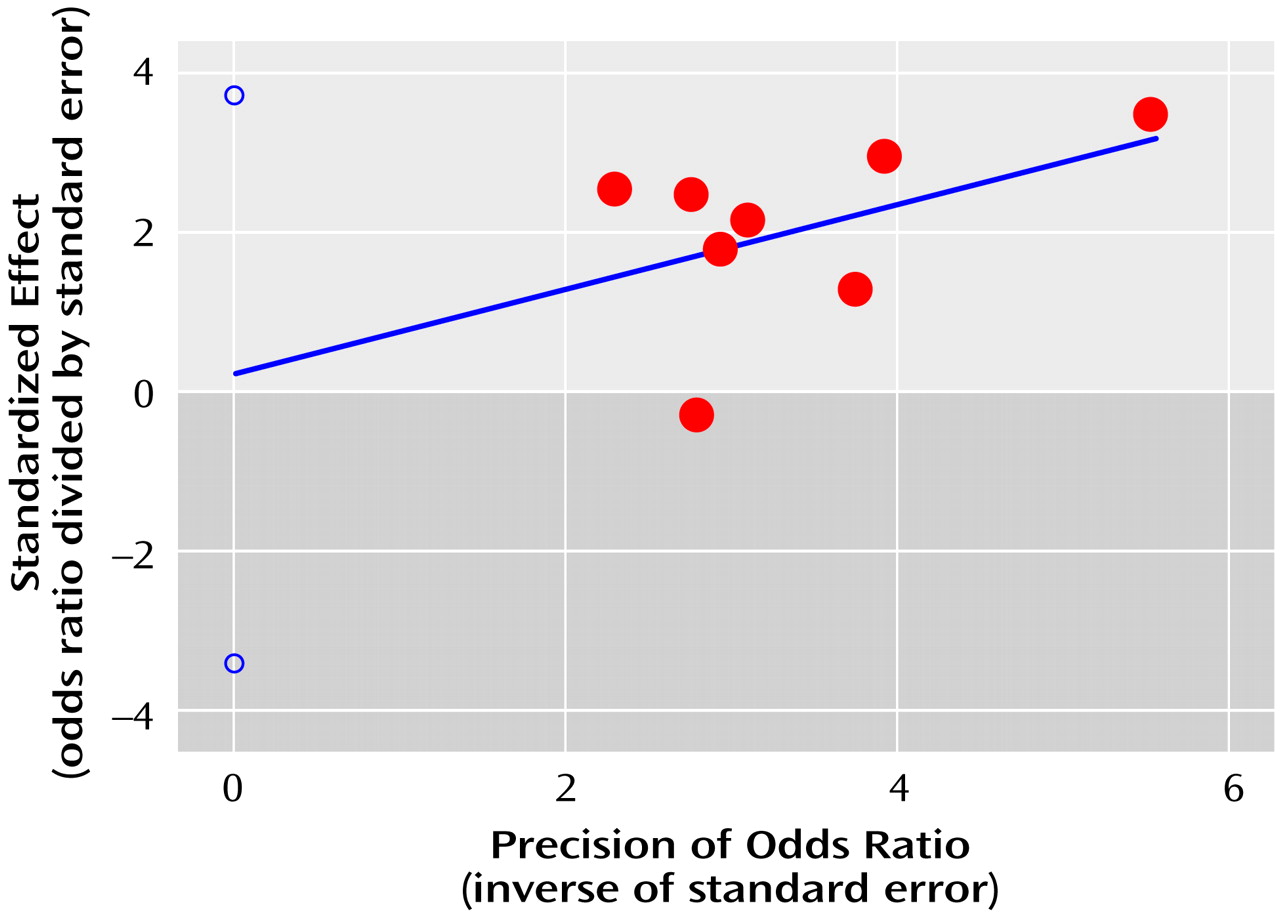
For the case-control studies, the publication bias statistic of Egger et al. was not significant (statistic=0.2, t=0.2, df=7, p=0.81), suggesting no publication bias. The regression plot determined by the method of Egger et al. (
Figure 1) shows the pattern expected for the absence of publication bias (see Method section).
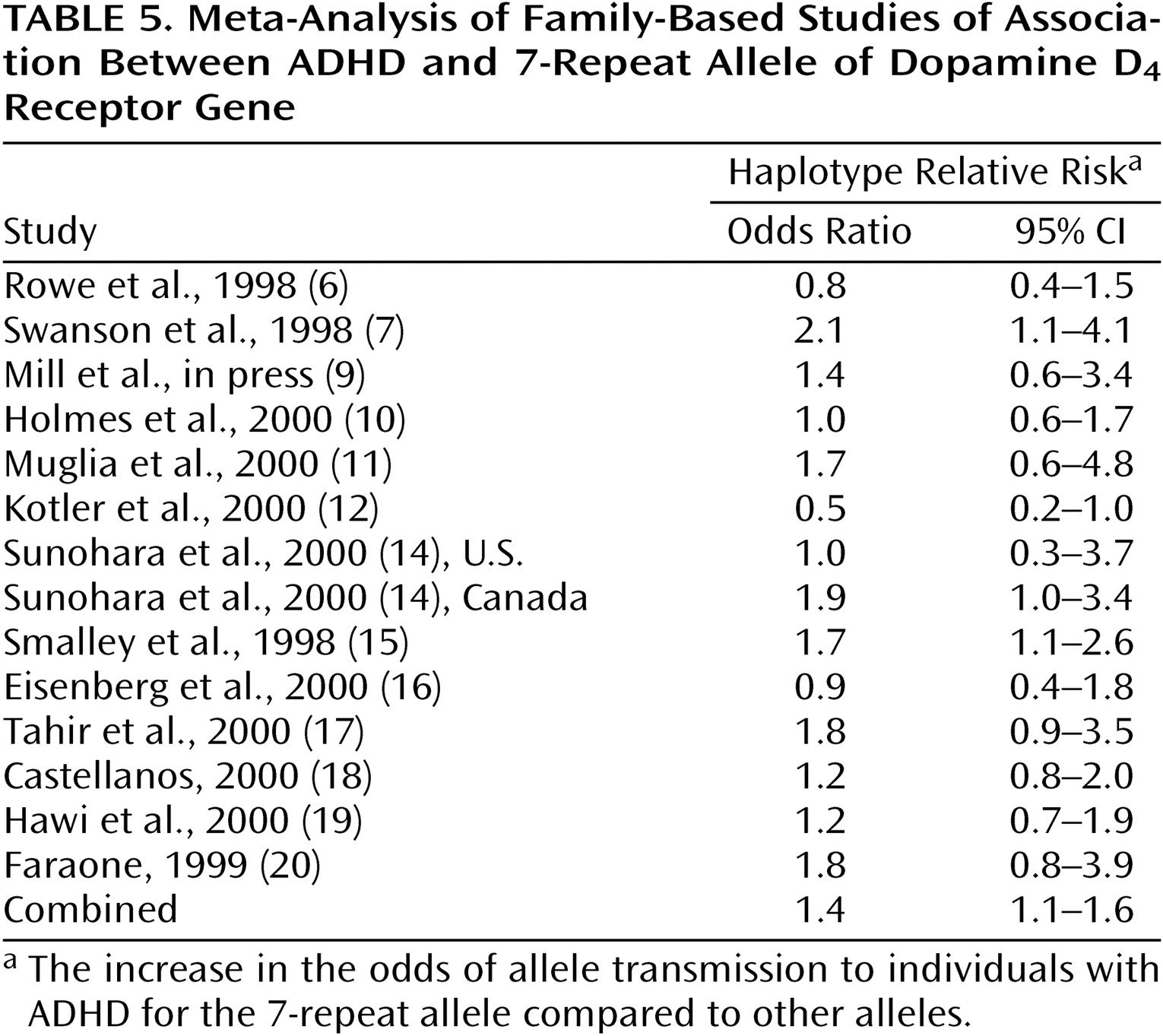
Table 5 gives the odds ratios and their 95% CIs for the 14 family-based studies. Although nine of these studies showed a positive association between ADHD and the DRD4 7-repeat allele, only two showed a statistically significant effect. The combined estimate was 1.4, and it was statistically significant (z=2.3, p=0.02, 95% CI for odds ratio=1.1–1.6). There was no statistically significant evidence for heterogeneity of the odds ratio among the family-based studies (χ
2=19.3, df=13, p=0.12). The population attributable risk was 0.09.
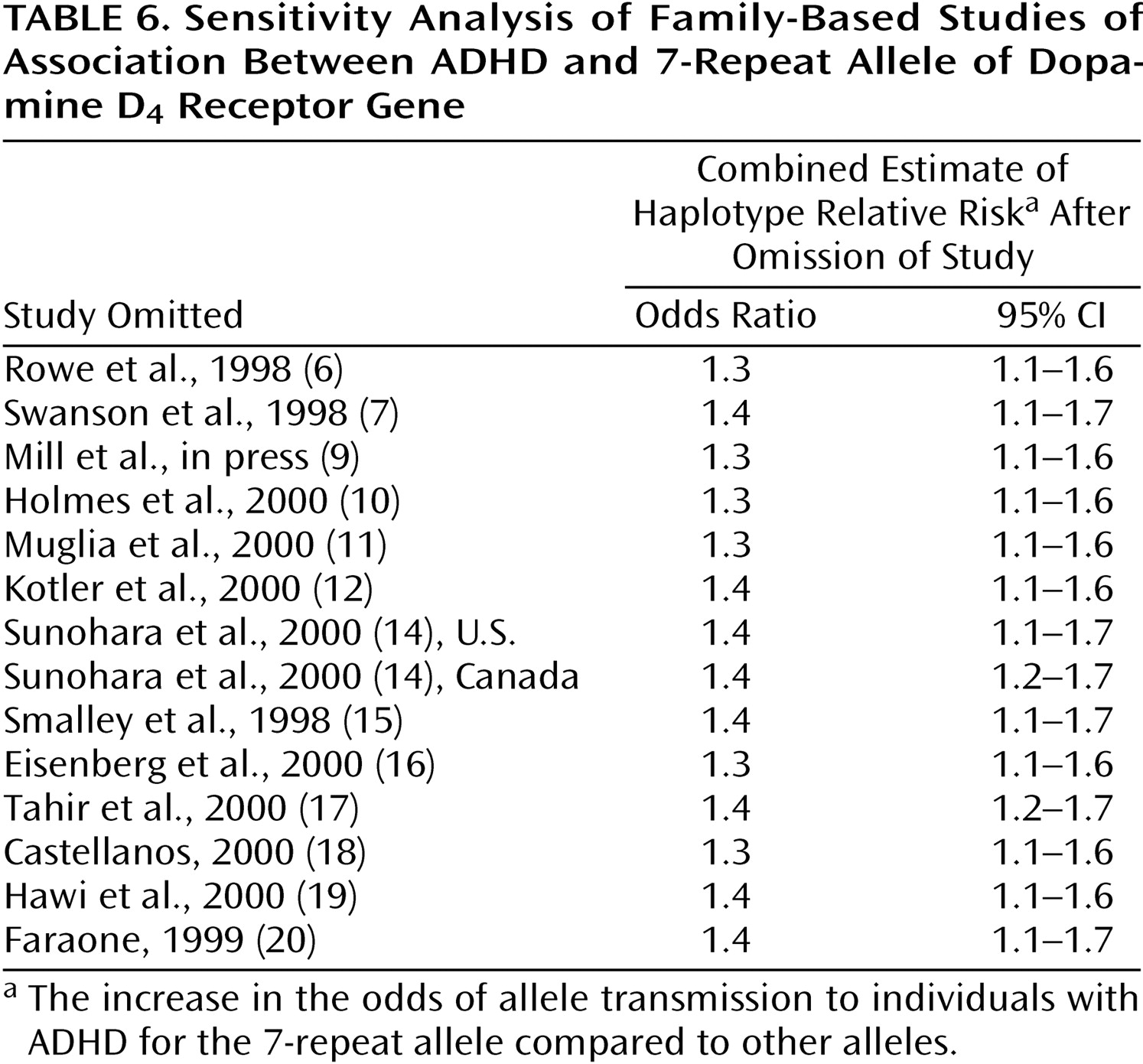
Table 6 shows the sensitivity analysis for the family-based studies. Estimates of the combined odds ratio range from 1.3 to 1.4, suggesting that no one study unduly influenced the combined estimate. As
Table 6 shows, the combined odds ratio was statistically significant regardless of which study is deleted.
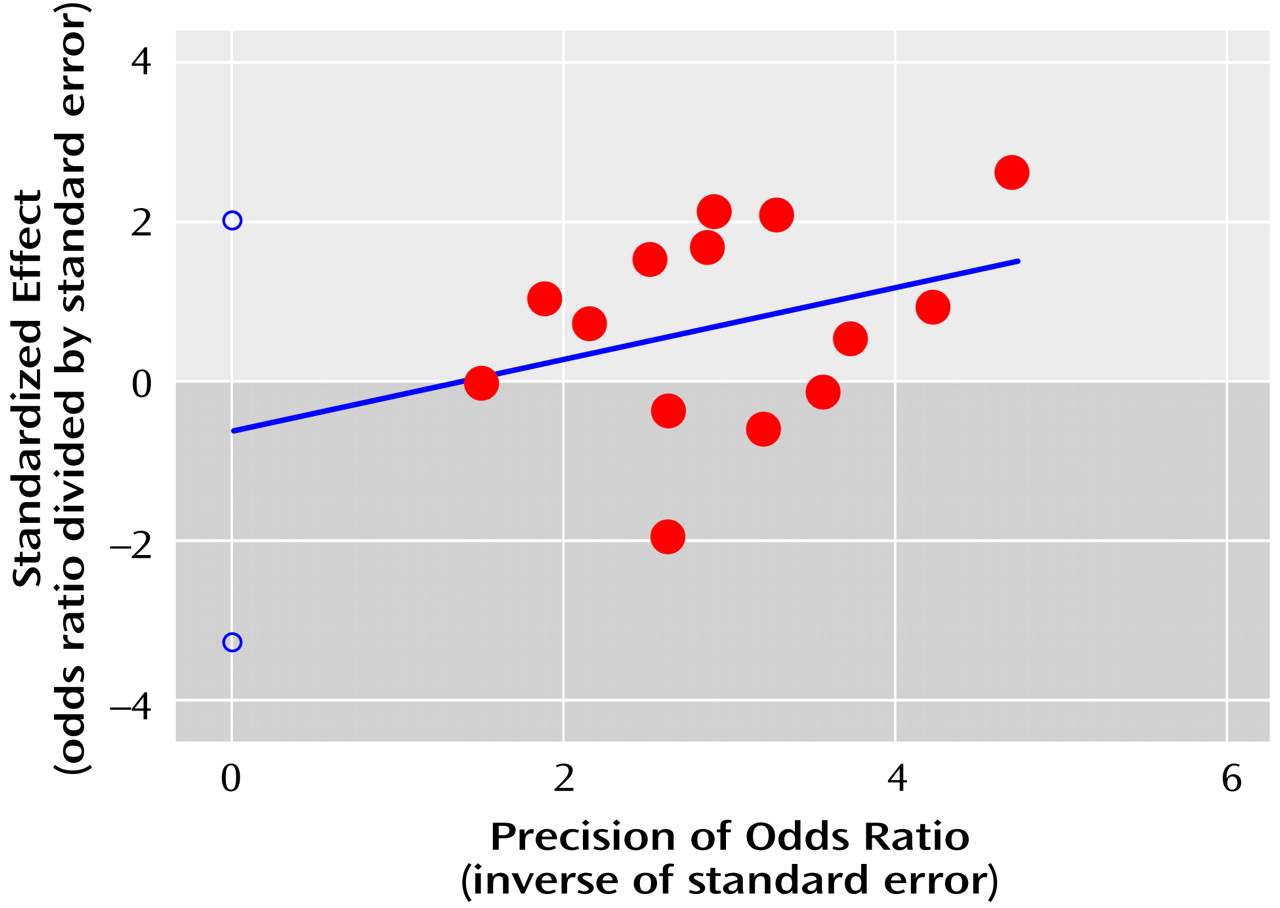
The publication bias statistic of Egger et al. was not significant (statistic=–0.6, t=–0.5, df=13, p=0.62), suggesting no publication bias for the family-based studies. The regression plot (
Figure 2) shows the pattern expected for the absence of publication bias.
Discussion
Our meta-analysis showed a small but statistically significant association between ADHD and the 7-repeat allele of DRD4. This association was statistically significant in separate analyses of eight case-control studies and 14 family-based studies. For each type of study, there was no evidence for heterogeneity of the odds ratio across studies, no evidence that a single study accounted for the significance or magnitude of the association, and no evidence for publication bias. Thus, our meta-analyses suggest that DRD4 is a susceptibility gene for ADHD.
The lack of heterogeneity among studies within each type of study is surprising given the wide range of clinical methods used (
Table 1 and
Table 2). Had we found statistical heterogeneity among studies, that finding would have attributed between-study differences to differences in methods of assessment, diagnosis, or ascertainment. But since we did not find statistical heterogeneity, it is reasonable to attribute differences among studies to chance fluctuations. This further suggests that it is appropriate to use meta-analysis to estimate a common odd ratios across studies.
Although there are about one-half as many case-control as family-based studies, the former studies provide much stronger evidence for the association between ADHD and DRD4. Notably, despite the significant meta-analysis findings, the results of only two of the 14 individual family-based studies were significant. Although counterintuitive, this likely reflects the low power of individual studies. This idea is supported by the fact that nine of the 14 fourteen studies had odds ratios greater than 1.0. Although the weaker result from the family-based studies can be seen as a confirmation of a prior hypothesis (i.e., the initial report of LaHoste et al.
[4]), the family-based data are less clear in asserting a significant association between ADHD and DRD4.
Case-control studies can be confounded by population admixture, and the prevalence of the 7-repeat allele is known to vary widely among ethnic groups
(28). Nonetheless, we have three reasons to believe that this confound cannot explain the apparent association between ADHD and DRD4. First, although it is possible that the findings from the case-control studies are spurious, we view this as unlikely because it would require us to assume a systematic bias in ascertainment across the eight case-control studies. Second, the haplotype-based haplotype relative risk method does not give false positive results in the presence of admixture. Instead, if there is heterogeneity in the frequencies of marker alleles among families, the haplotype-based haplotype relative risk will provide a conservative statistical test
(29). Third, if DRD4 confers susceptibility to ADHD, there will be zero recombination between marker and disease gene, and the odds ratios from the studies with case-control and haplotype-based haplotype relative risk designs should be identical
(24). Although there is a small difference between the two, it is not significant according to the overlap in their 95% CIs.
Other considerations suggest that DRD4 plays a role in the etiology of ADHD. Both noradrenaline and dopamine have been implicated in the pathophysiology of ADHD through animal models and human treatment studies
(1). Both of these neurotransmitters are potent agonists of DRD4
(30). In vitro studies
(31,
32) showed that the DRD4 7-repeat allele mediates a blunted response to dopamine, although the biological significance for ADHD is not clear given the small effects found in these studies
(33). In addition, the distribution of DRD4 mRNA in the brain suggests it plays a role in cognitive and emotional functions, functions implicated in the pathophysiology of ADHD
(33).
A link between DRD4 and one of the core features of ADHD, hyperactivity, was implicated by a “knockout” mouse study
(34). When DRD4 was disabled in mice, dopamine synthesis increased in the dorsal striatum, and the mice showed locomotor supersensitivity to ethanol, cocaine, and methamphetamine. DRD4 knockout mice also show reduced novelty-related exploration
(35), which is consistent with human data suggesting a role for DRD4 in novelty-seeking behaviors.
Despite these considerations, we cannot say for sure that the DRD4 7-repeat allele confers susceptibility to ADHD. The association could be due to an unknown gene in linkage disequilibrium with DRD4. Even if DRD4 confers susceptibility to ADHD, it is possible that a DRD4 variant other than the 7-repeat allele accounts for the observed association with ADHD
(36).
Consistent with the idea that ADHD is a complex, multifactorial disorder, the magnitude of the association between DRD4 and ADHD is small. This can be seen in the low odds ratios and the correspondingly small attributable risks, which suggest that other genes work in concert with DRD4 to cause ADHD. Despite this small effect size, future studies of DRD4 are warranted to determine what variants of DRD4 or a nearby gene increase susceptibility to ADHD.