Family, twin, and adoption studies show that bipolar disorder is highly heritable
(1). Nevertheless, efforts to identify susceptibility genes for bipolar disorder have led to largely inconclusive results
(2). The lack of conclusive findings may be attributable in part to the fact that bipolar disorder is a common disease with complex inheritance
(3). An obstacle to identification of genes for bipolar disorder and other heritable psychiatric disorders is the lack of resolution of the affected phenotype, leading to a lumping together of etiologically heterogeneous disorders
(4). Although psychiatric disorders probably represent final common pathways for different causal processes
(4) and it is unlikely that improved definition of the phenotype will create a Mendelian disorder from a complex psychiatric disorder, insufficient progress has been made in the refinement of the psychiatric phenotype for the purpose of reducing genetic heterogeneity
(5).
There is evidence from genetic epidemiologic data and even some linkage data that bipolar disorder that is comorbid with panic disorder may define a genetic subtype of bipolar disorder. In two family studies, De Paulo and co-workers
(6,
7) found that panic disorder had a significantly higher prevalence in first-degree relatives with bipolar disorder in the family of probands with comorbid bipolar disorder and panic disorder, compared to relatives of bipolar disorder probands without panic disorder. The same authors reanalyzed their bipolar disorder linkage data in a data set that showed linkage to chromosome 18q and that was stratified for the presence or absence of familial comorbid panic disorder
(8). They found stronger statistical evidence for linkage to 18q in the families of probands with comorbid bipolar disorder and panic disorder than in those with bipolar disorder without panic disorder
(8). These findings are consistent with the hypothesis that panic disorder is an index of genetic heterogeneity in bipolar disorder.
We hypothesized that comorbidity for panic disorder may also influence the strength of associations between bipolar disorder and three candidate genes implicated by their role in monoamine neurotransmission. If comorbid panic disorder identifies an etiologically distinctive bipolar disorder subgroup, we expected that one or more of these genetic associations would be strengthened when individuals with bipolar disorder were stratified by the presence of panic disorder. The three candidate genes we considered were catechol
O-methyltransferase (COMT)
(9,
10) serotonin transporter (5-HTT) promoter
(11–
13), and tryptophan hydroxylase (TPH)
(14).
Genetic variation in COMT, which metabolizes dopamine, norepinephrine, and epinephrine, could be important in modulating the diverse functions in which these monoamines are involved. The COMT Val158Met polymorphism is common, with a rare allele frequency of about 0.4; subjects with the low-activity Met158 allele have a three- to fourfold lower level of enzymatic activity and a lower level of thermal stability than subjects without this allele
(15,
16). Significant association with bipolar disorder has been reported for the low-activity allele Met158 in the Han Chinese population
(9) and in rapid cycling bipolar disorder patients
(10). Furthermore, there is substantial evidence from linkage studies for a susceptibility locus for bipolar disorder in the chromosome 22 q11–13 region, which includes the COMT gene
(17,
18).
A functional 44-base-pair insertion/deletion polymorphism was detected in the promoter region of the serotonin transporter (5-HTT) gene
(19). The short allele (484 base pairs) of the 5-HTT gene-linked polymorphic region (5-HTTLPR) is associated with a lower level of transcriptional efficiency of the 5-HTT promoter in vitro
(19), and genotype-determined differences in the in vivo expression of 5-HTT have also been reported
(20). Two independent investigations
(11,
12) and a meta-analysis
(13) showed weak evidence of genetic association between the 5-HTTLPR low-activity allele and bipolar disorder.
In this report we attempt to clarify the relationship of these three monoaminergic candidate genes to bipolar disorder by using comorbid panic disorder as a grouping variable to better specify the bipolar disorder phenotype and, potentially, to reduce heterogeneity in the etiology of bipolar disorder.
Discussion
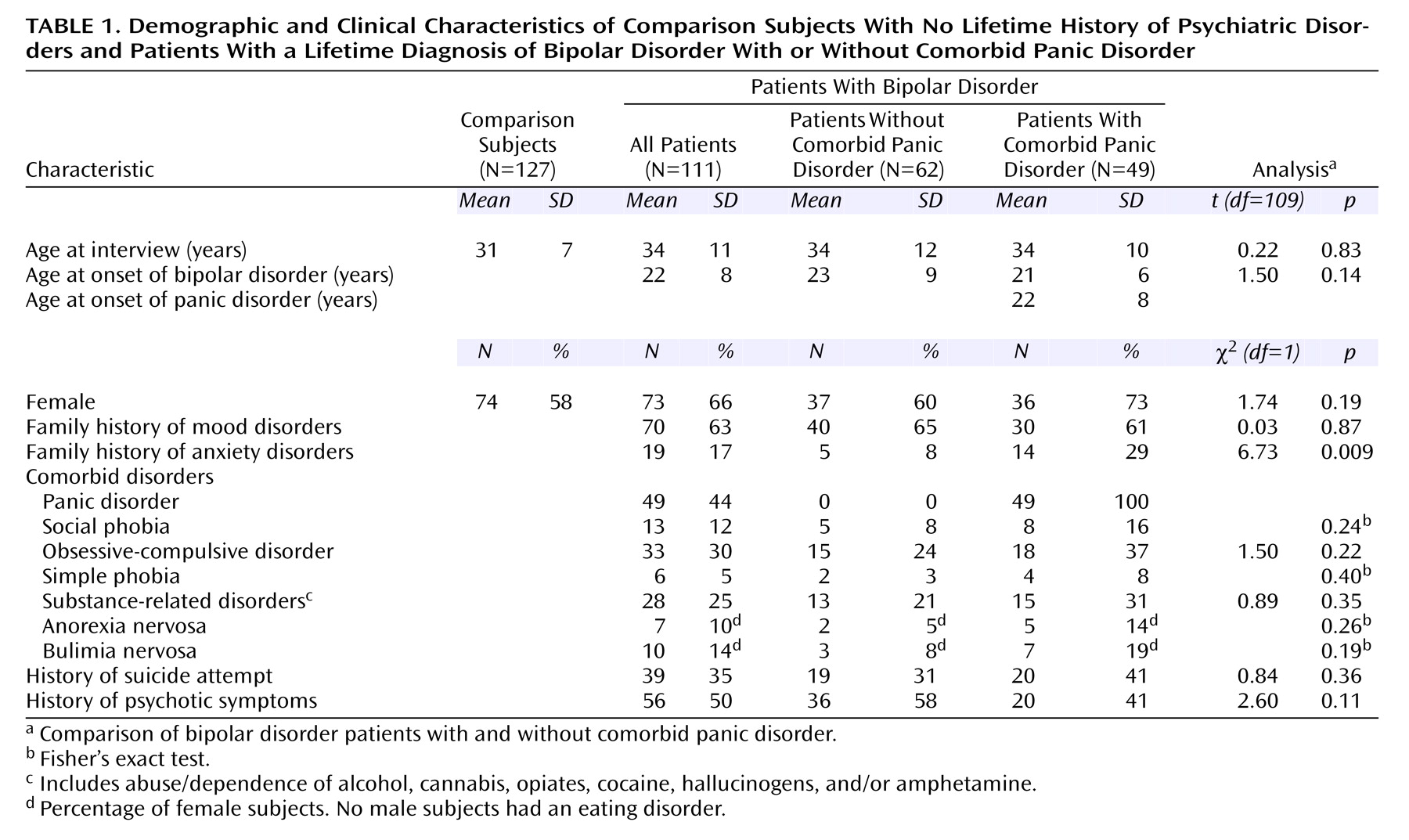
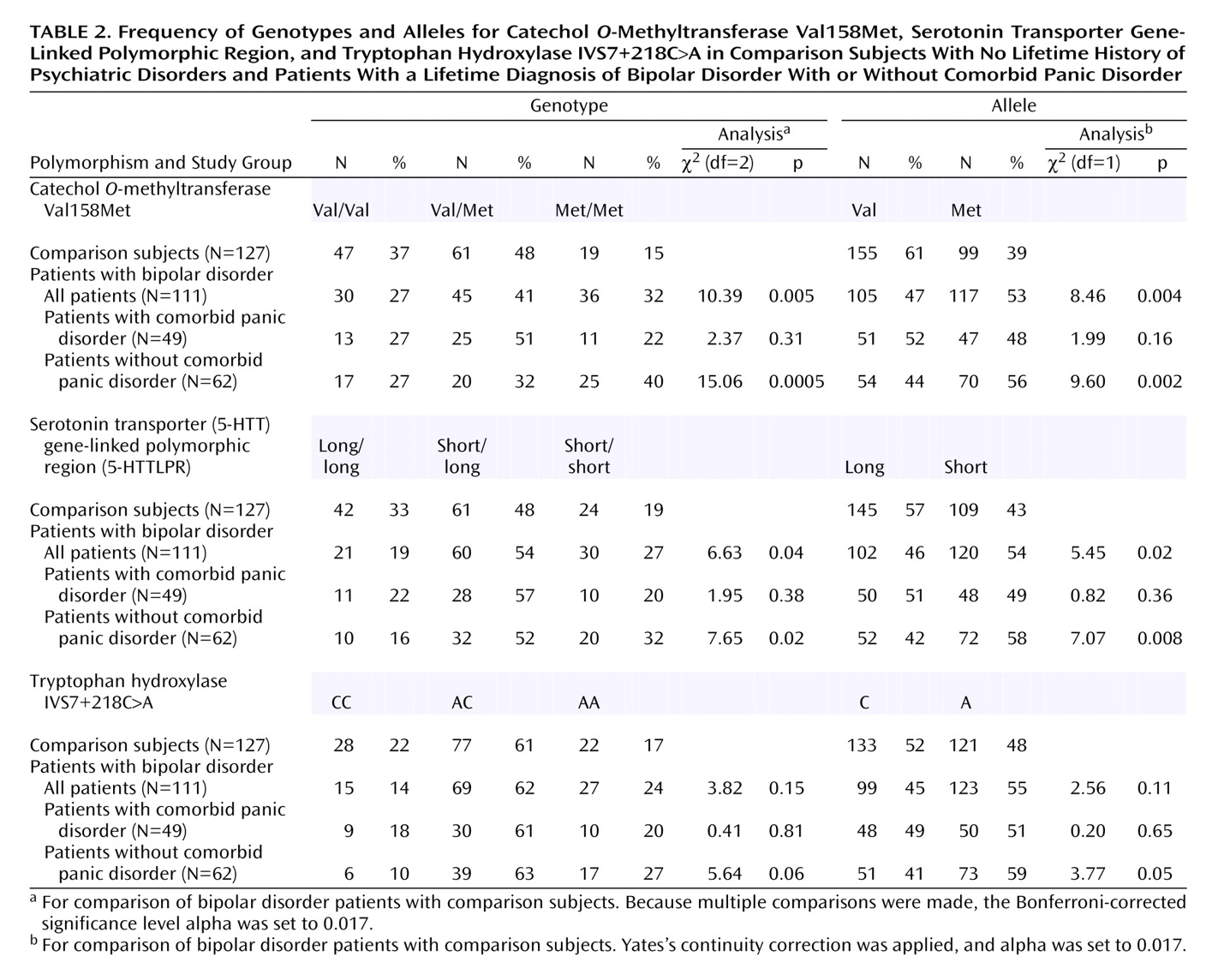
Using a case-control association strategy, we found evidence that panic disorder is a marker of genetic heterogeneity in bipolar disorder, strengthening already significant associations of functional COMT and serotonin transporter polymorphisms with bipolar disorder. Our findings indicate strong genotypic and allelic association of two independent variants of COMT and 5-HTT genes with bipolar disorder in the absence of comorbid lifetime panic disorder. These associations remained statistically significant even after Bonferroni correction. Associations of these variants with comorbid bipolar disorder and panic disorder were more modest and did not withstand correction for multiple comparisons. Although clinical variables other than panic disorder (e.g., age at onset of bipolar disorder, diagnoses comorbid with bipolar disorder, suicidality, and presence of psychotic symptoms) could in theory mediate an association between bipolar disorder and the genes analyzed, we decided not to analyze the relationship of these clinical characteristics and the genotype and allele distributions to avoid inflation of type I error. In agreement with recent family data
(6,
7), we also found that anxiety disorders were significantly more prevalent in families of subjects with comorbid bipolar disorder and panic disorder than in those of subjects with bipolar disorder without panic disorder. This finding should be considered with caution, as the family information was collected from probands only
(33). However, it is consistent with a difference in familial vulnerability between these two bipolar disorder phenotypes.
To our knowledge, this is the first use of panic disorder as a grouping variable in a case-control association study of bipolar disorder. Taken together, our findings provide further support for the genetic epidemiologic
(6,
7) and linkage
(8) evidence suggesting that comorbid panic disorder is a marker for a distinct genetic subtype of bipolar disorder. Epidemiological and clinical findings also raise suspicion that the comorbidity between the two disorders is the manifestation of a specific phenotype. Epidemiological studies suggest that comorbidity rates between bipolar disorder and panic disorder were higher than could be expected by chance alone. In the Epidemiologic Catchment Area study in the United States, the lifetime prevalence of panic disorder among subjects with bipolar disorder was 20.8%, 26 times higher than in subjects without any other axis I disorder (0.8%) and 2.1 times higher than in individuals with unipolar depression (10.0%)
(34). In agreement with these findings, the National Comorbidity Survey, a general population survey of DSM-III-R disorders in the United States, reported that 33.1% of subjects with bipolar disorder had comorbid panic disorder
(35) and were at greater risk for panic disorder than subjects with unipolar depression
(36). The lifetime prevalence of panic disorder among bipolar disorder subjects in clinical study groups is even higher, as high as 62.5%
(37–
40). Clinically, it has been suggested that panic disorder and high anxiety levels in the context of bipolar disorder predict greater severity, poorer prognosis, and resistance to pharmacological treatments
(38,
41,
42).
On the basis of the significant results reported here, bipolar disorder without panic disorder may represent a more homogeneous form of the illness, genetically distinct from bipolar disorder with panic disorder and more strongly related to the function of COMT Val158Met and 5-HTTLPR. These variants may influence specific clinical features of bipolar disorder independent of those affecting liability
(43). Therefore, the association of both of these polymorphisms with a genetic subtype of bipolar disorder, such as bipolar disorder without panic disorder, may be better understood as a relationship of alleles to a modifying trait for various psychiatric diseases, rather than a one-to-one causative relationship between functional alleles and a particular psychiatric disease
(43).
The success of the clinical subgrouping in this study may partly explain the inconsistent results for the association of COMT and 5-HTT variants with the broadly defined bipolar disorder phenotype, since it is likely that the frequency of panic disorder differs in different groups of bipolar disorder patients. Thus far, several studies of the COMT Val158Met polymorphism in bipolar disorder have had negative findings
(27–
29,
44,
45), although a positive association was found between the low-activity Met158 allele and bipolar disorder in a Han Chinese population
(9) and in a group of rapid cycling bipolar disorder patients
(10). The COMT gene falls within the region of chromosome 22q11, which has been indicated among possible susceptibility loci for bipolar disorder in linkage studies
(17,
18). On the basis of the earlier family
(6,
7) and linkage
(8) findings and the current results, we suggest that stratification of chromosome 22 linkage data by familial comorbid panic disorder could be useful for increasing the resolution of linkage analysis. As for 5-HTTLPR, a number of studies failed to find a role for this polymorphism in bipolar disorder
(13,
30,
31,
46–
48), although two independent investigations
(11,
12) and a meta-analysis
(13) found weak evidence for a genetic association between the 5-HTTLPR low-activity allele and bipolar disorder. Finally, it is also possible that the association between the TPH IVS7+218C>A variant and bipolar disorder reported by Bellivier and coworkers
(14), while not replicated elsewhere
(32,
48–
50), could be strengthened and eventually validated with the aid of clinical subgrouping.
The strength of our conclusions may be mitigated by several methodological limitations. Although the size of the study group was fairly large in comparison to those in many association studies in neuropsychiatry, statistical power to detect potentially important effects may have been limited. For example, for a susceptibility allele present in comparison subjects with a frequency of 0.4, we could detect genotypic relative risks of approximately 2.7 under a dominant model and 2.8 under a recessive model with 80% power (assuming two-tailed alpha=0.05) for the critical comparisons between the group with comorbid bipolar disorder and panic disorder and the comparison group or between the bipolar disorder patients without panic disorder and the comparison group. Therefore, further studies with larger study groups are needed to draw definitive conclusions.
A possible limitation in case-control association studies is the risk of spurious associations as a result of population admixture
(51). However, this objection is less likely in the present study, as our study group was composed of subjects of Italian descent for generations and because the Italian population is relatively homogeneous
(52). We also note that the COMT and HTTLPR allele frequencies observed in the Italian comparison subjects are similar to the allele frequencies found in diverse Caucasian populations.
Despite these limitations, our report provides additional evidence for a role for two loci in bipolar disorder and for the existence of subgroups of bipolar disorder indexed by comorbidity with panic disorder. Future research involving larger study groups will be required to determine whether a clinical subtype of bipolar disorder exists and, if so, whether it represents a more homogeneous phenotype for genetic studies. To finally exclude cryptic population stratification as the source of the observed association, replication studies with a family-based design and control loci to formally evaluate stratification may be employed.