Anorexia nervosa is a significant and growing health problem, with peak ages of onset at 12–14 and at 18
(1). With a prevalence of 1%, it is the third most common chronic disorder in adolescent girls, after obesity and asthma
(2). The prevalence for individuals who are subthreshold for the disorder is, however, significantly higher. Anorexia nervosa is also highly resistant to treatment. Depending on criteria, recovery varies between 23% and 50% and relapse from 4% to 27%
(3). Mortality is estimated to be between 6% and 20%, depending on length of follow-up
(3,
4).
Practice guidelines urge a multidisciplinary approach in the treatment of anorexia nervosa, with recommendations regarding nutritional rehabilitation, psychotherapeutic interventions, and psychotropic interventions
(4). Although the role of psychotherapeutic and psychotropic interventions is being explored
(5), there has been little attention accorded to innovation in nutritional rehabilitation. In the United States, the standard nutritional rehabilitation protocol in the treatment of anorexia nervosa has been—and remains—staged oral refeeding.
This lack of research on nutritional interventions is surprising for several reasons. First, weight restoration is associated with an attendant decrease in key anorectic symptoms and an improvement in physical and cognitive function
(4). Second, weight restoration is a prerequisite for the effective use of psychotherapeutic and psychotropic interventions
(4,
5). Third, most patients with anorexia nervosa suffer from delayed gastric emptying and impaired intestinal motility
(6). Thus, they experience intense physical distress as well as intense psychological distress when they must eat the large amount of food needed for weight restoration and recovery. Fourth, with the advent of managed care, there has been greater pressure to transfer patients from inpatient care to less intensive treatment relatively early in their course of treatment and usually at a suboptimal weight
(7). Finally and most compelling, low discharge weight is a critical risk factor in relapse and rehospitalization
(7–
10).
The standard nutritional rehabilitation protocol for oral refeeding appears to be limited in its effects. It is timely to explore alternative feeding practices that may enhance weight restoration in anorectic patients. One such alternative may be the use of nasogastric refeeding to complement oral refeeding.
The use of nasogastric refeeding in the treatment of patients with anorexia nervosa has a history of controversy, with objections raised on both clinical and ethical grounds. Nasogastric refeeding is viewed as a means of forced feeding, and clinical lore holds that forced feeding may place patients with anorexia nervosa at greater risk for relapse, for developing complications (e.g., bulimia), and in general, for more negative long-term outcomes
(11). However, there is no empirical documentation of these concerns. The controversy appears to have been founded in anecdotal accounts of clinical experience with methods of forced feeding other than nasogastric refeeding, with severely morbid cases of anorexia nervosa, when nasogastric refeeding was the treatment of last resort, when it was the exclusive means of caloric intake, and when it was used in the absence of psychotherapeutic interventions
(12,
13). Bruch
(13), for example, reported deterioration in three severely anorectic patients who had been denied all activities and amenities (e.g., contact with others, books, etc.) as a means of forcing them to eat.
Nasogastric refeeding, in fact, has a history of safe and effective use in the treatment of pediatric patients with diseases that are characterized by poor oral intake and/or higher caloric requirements. These diseases include Crohn’s disease
(14), cancer
(15), and cystic fibrosis
(16). In addition, for the past two decades, nasogastric refeeding has been used safely and effectively in several European countries to treat anorexia nervosa
(17).
There have also been objections to the use of nasogastric refeeding in patients with anorexia nervosa on complex legal and ethical grounds. These issues have been addressed extensively by others
(18,
19) and are perhaps most relevant in instances when patients actively resist or refuse treatment. It is also widely believed that patients experience nasogastric refeeding as invasive and coercive. While this may in fact be the case, it remains an issue in need of systematic study. The lone study that exists—a retrospective self-report
(20)—found that patients’ and parents’ responses ranged from fairly positive to wholly negative; however, the majority of respondents indicated that they believed that the use of nasogastric refeeding had been essential and that there had been no feasible alternative at the time of treatment.
We advocate a nutritional rehabilitation protocol that remains firmly grounded in staged oral refeeding but also includes supplemental nocturnal nasogastric refeeding. Nocturnal feeding allows for a gradual increase in calories consumed over 24 hours. A nocturnal supplement, administered slowly over time, will not produce as much pain and distension in patients with anorexia nervosa as either oral food intake or rapid nasogastric replacement. With this approach, daytime oral intake is neither replaced nor decreased, and daytime appetite is allowed to return. The nasogastric tube is removed once the patient reaches 95% of his or her ideal body weight, and by the time of discharge, the oral caloric intake is at a maintenance level.
Since October 1995, nocturnal nasogastric refeeding has been our standard of care in the nutritional rehabilitation of patients who meet the following criteria: 1) a primary diagnosis of anorexia nervosa, 2) weight at or below 85% of ideal body weight, and 3) no physiological contraindication to nasogastric refeeding (e.g., esophageal tear or rupture).
Consistent with our conceptualization of anorexia nervosa as not only a psychiatric disorder but also a disorder with important medical-biological components, we demystify and medicalize nocturnal nasogastric refeeding when we explain it to parents and patients. Essentially, we present it as a time-limited medical procedure to aid in the physiologic recovery of the digestive system and in emotional adjustment. We explain that it is our standard of care for patients who meet the criteria and also extensively discuss its risks and benefits. This approach offers little or no context for nocturnal nasogastric refeeding to become an issue for bargaining, delay, or stigmatization. It also obviates the possibility that the treatment in and of itself is perceived as coercive. Moreover, because the nasogastric tube is placed at admission, it cannot be viewed as a punishment for not following the oral refeeding protocol.
The following is a brief synopsis of the nocturnal nasogastric refeeding procedures that we have implemented. Nursing staff places the nasogastric tube and verifies placement of the tube in the stomach by auscultation, pH level, or X-ray. This tube is typically placed on admission and remains in place throughout nocturnal nasogastric refeeding. On occasion, however, it may be necessary to remove the nasogastric tube because of nasal irritation. In this case, another tube is placed through the other nostril. The nutritional supplement contains 1.5 kcal/cc and is delivered overnight by Kangaroo 324 feeding pump (Sherwood Medical, St. Louis). The ratio of daytime oral caloric intake to caloric intake through nocturnal nasogastric refeeding is approximately 2:1. The number of calories administered through nocturnal nasogastric refeeding is slowly increased over the first 3 nights. The first night, the rate of caloric intake through nocturnal nasogastric refeeding is 40 cc/hour for 4 hours then 60 cc/hour for 4 hours (total of 600 kcal). The second night, the rate is increased to 80 cc/hour for 4 hours then 100 cc/hour for 4 hours (1080 kcal). The third night, and all subsequent nights, the rate is 100 cc/hour for 8 hours (1200 kcal). Nocturnal nasogastric refeeding is discontinued approximately 3–4 days before discharge or earlier if the patient achieves 95% of his or her ideal body weight. The transition to caloric intake by oral refeeding only is gradual. Nocturnal nasogastric refeeding is reduced by 2 hours (200 cc or 300 kcal) each night, with a commensurate increase in daytime oral caloric intake.
The primary medical concern in nocturnal nasogastric refeeding is the potential for hypophosphatemia and/or hypomagnesemia. This is not unique to nocturnal nasogastric refeeding; it is the case with any refeeding protocol
(21). We carefully monitor patients to prevent problems. Heart rate, nutritionally important laboratory test results, and dependent edema are continually monitored, and oral phosphorus is promptly administered if indicated.
Nocturnal nasogastric refeeding to supplement oral refeeding may be a viable alternative to standard oral refeeding in the treatment of patients with anorexia nervosa. Thus, it is important to examine its short-term and long-term risks and benefits. We are not aware of any previous studies that have done so. In this study, we compared the short-term outcomes of the standard nutritional rehabilitation protocol—staged oral refeeding—with a nutritional rehabilitation protocol that used supplemental nocturnal nasogastric refeeding to complement staged oral refeeding. Specifically, it was hypothesized that at discharge the nocturnal nasogastric refeeding group would show a significantly greater increase in weight and body mass index than the oral refeeding group. It was also predicted that length of hospital stay would be significantly shorter for the nocturnal nasogastric refeeding group.
Method
Subjects
A retrospective chart review was conducted at an academic pediatric hospital located in a major eastern U.S. city. Approval to review records was obtained from the hospital’s institution review board. Subjects in this study were patients admitted between June 1, 1992, and December 31, 1998. For inclusion, subjects were required to meet strict DSM-IV criteria for diagnosis of anorexia nervosa without also meeting criteria for bulimia nervosa. Subjects were not further classified into anorexia nervosa subtypes (restricting or binge-purge types) because in a subset of cases data were insufficient to retrospectively make this distinction with validity.
Of 158 patient medical records initially reviewed for this study, 125 patients met the diagnostic criteria. Of these, six patients were boys and were excluded. Of the 119 girls, 18 were non-Caucasians and were also excluded because their small numbers did not allow for analysis of race effects. Finally, one Caucasian girl was excluded because of missing data. Thus, the study group consisted of 100 Caucasian girls.
The 100 subjects who met inclusion criteria were grouped according to the nutritional rehabilitation protocol that they received: either staged oral refeeding or supplemental nocturnal nasogastric refeeding complementing staged oral refeeding. The oral refeeding group consisted of 48 subjects (mean age=15.0 years, SD=1.8), and the nocturnal nasogastric refeeding group consisted of 52 subjects (mean age=14.8 years, SD=1.9).
Data Collection
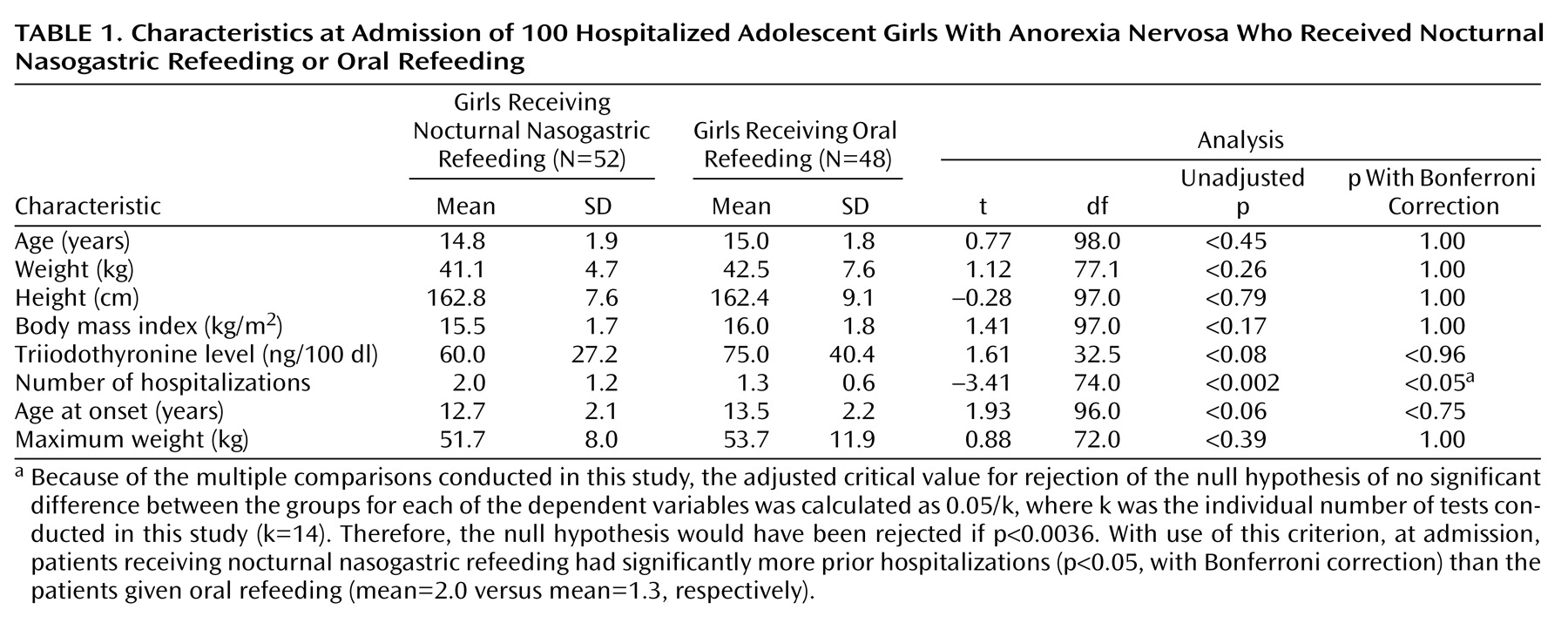
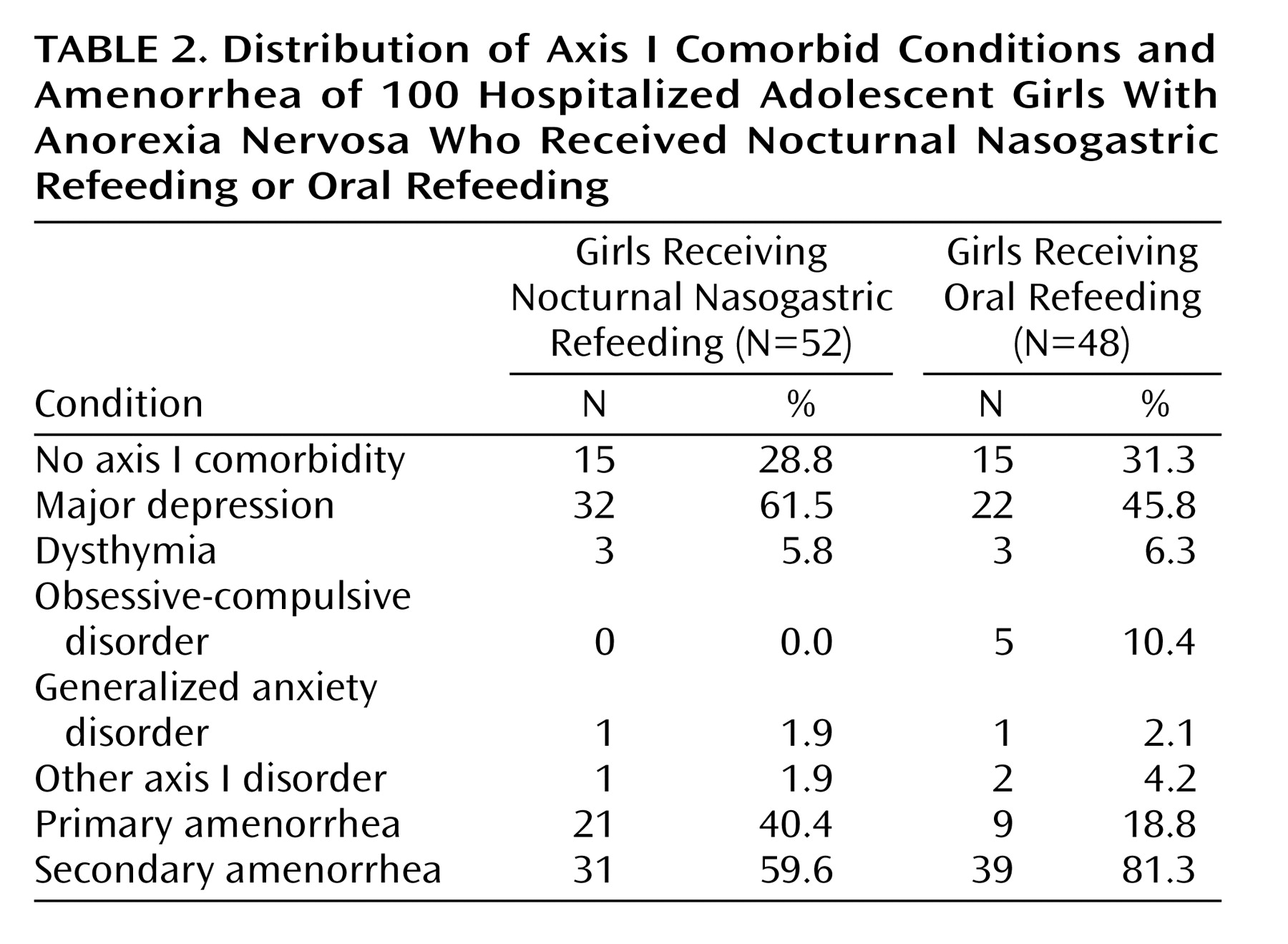
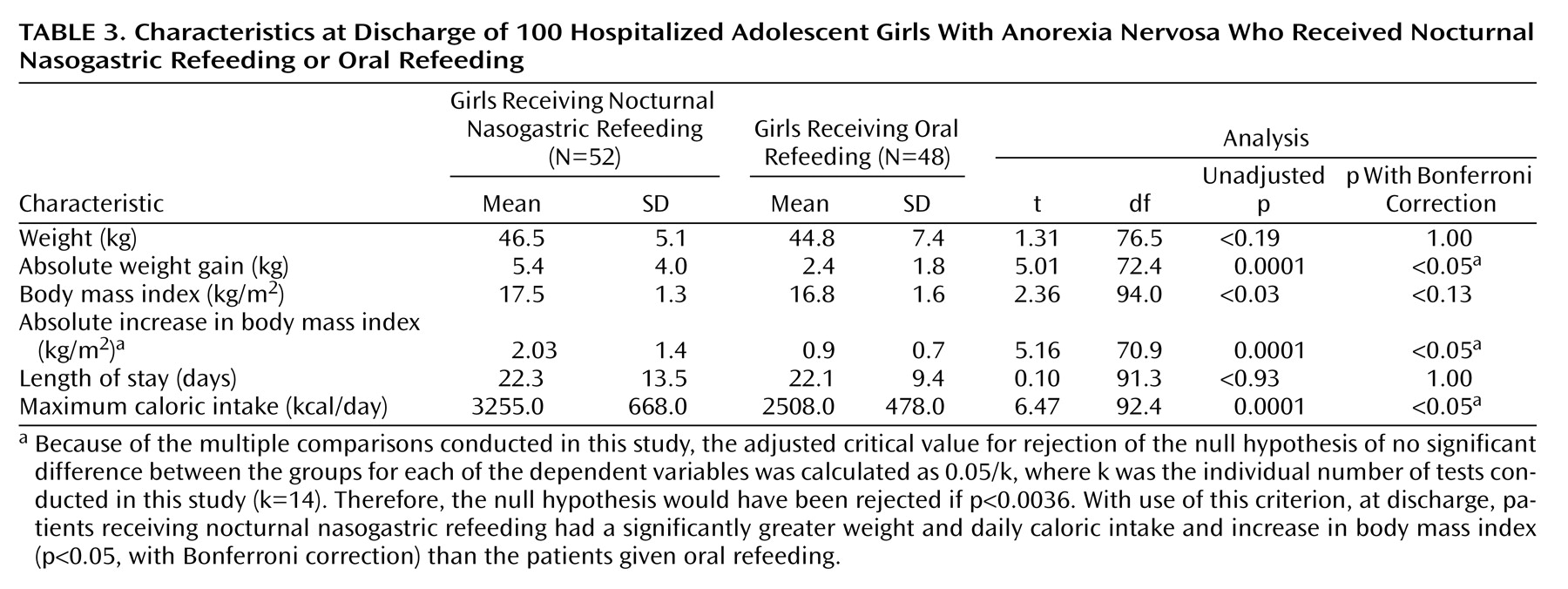
Data were collected from a retrospective review of patients’ medical records, including admission notes, inpatient progress notes and orders, discharge summaries, and outpatient records. A child psychiatrist reviewed the charts to retrieve data on relevant demographic, diagnostic, and anthropometric variables (
Table 1,
Table 2, and
Table 3). These data were then verified, first by another child psychiatrist and then by a child psychologist. When there was disagreement, it was discussed and resolved among the three researchers (A.S.R., N.E., M.J.D.). Because this procedure yielded a single record of the data, an estimate of reliability was not possible.
Treatment
Oral refeeding protocol
The 48 subjects in the oral refeeding group received the staged oral refeeding protocol, daily group therapy, thrice-weekly individual therapy, twice-weekly family therapy, and twice-weekly expressive therapy. However, the patients in this group were not all treated in the same setting. Twenty of the 48 patients were treated on the adolescent medicine unit with psychiatric consultation. The remaining 28 oral refeeding patients were initially treated on the adolescent medicine unit with psychiatric consultation until medical stabilization. They were then transferred to the (then) newly established adolescent psychiatric unit for psychiatric treatment with adolescent medicine consultation. The adolescent psychiatric unit admits patients aged 13–19 with a primary psychiatric disorder, excluding only violent patients and patients with alcohol and/or substance addictions. On the adolescent psychiatric unit—but not on the adolescent medicine unit—patients had continuous access to specialized psychiatric nursing staff, took meals as a group, and participated in planned group activities.
The standard oral refeeding protocol for patients with anorexia nervosa was administered to this group. This treatment consists of a graduated behavioral refeeding program designed to promote nutritional rehabilitation. Calories are consumed in three meals and two snacks and are gradually increased as tolerated to maintain a weight gain of approximately 1 to 2 kg/week. Patients are monitored for refeeding syndrome
(21). Meals must be eaten within a given time period, and all calories not consumed as part of the meal must be replaced with a calorie-dense nutritional supplement by mouth immediately after the meal. Exercise is not allowed until patients are compliant with meals and are above 85% of their ideal body weight. As part of the transition to partial hospitalization or outpatient care, patients are given more responsibility in the selection and consumption of meals and practice eating outside the hospital with their families. The goal for weight at discharge is at least 95% of the ideal body weight.
Nocturnal nasogastric refeeding
All 52 patients in the nocturnal nasogastric refeeding group were treated on the adolescent medicine unit until medical stabilization, then they were transferred to the adolescent psychiatric unit. All received the nocturnal nasogastric refeeding protocol as well as the oral refeeding protocol. Like the oral refeeding group, this group received daily group therapy, thrice-weekly individual therapy, twice-weekly family therapy, and twice-weekly expressive therapy. However, this group also had continuous access to specialized psychiatric nursing staff, took meals as a group, and participated in planned group activities.
Outcome Variables
The primary outcome variables in this study were weight change, change in body mass index, and length of hospital stay. Weight was measured in kilograms. Change in weight was calculated by subtracting weight at admission from weight at discharge. Body mass index was calculated as weight in kilograms divided by height in meters squared. Length of hospital stay was calculated in terms of days hospitalized.
Data Analyses
The Statistical Analysis System
(22) was used in these analyses. All statistical tests were two-tailed. Preliminary analysis of differences between the two treatment groups, without adjustment for potential confounding factors, involved use of unpaired t tests with Bonferroni correction for multiple comparisons. These tests were used to determine whether significant differences existed between the two treatment groups at the time of admission (
Table 1) and at discharge (
Table 3). Multivariate linear regression was used to establish the independent effects of nocturnal nasogastric refeeding on outcome variables at discharge, with simultaneous control for potential confounding variables. In the multivariate models, type of treatment (nocturnal nasogastric refeeding or oral refeeding) and location of treatment (adolescent medicine unit or adolescent psychiatric unit) were coded as dichotomous variables, with oral refeeding and adolescent psychiatric unit, respectively, serving as the referents; all other predictor variables were coded as continuous.
Results
The two groups did not differ significantly in age, weight, height, body mass index, triiodothyronine level, age at onset, or maximum lifetime weight at time of admission (
Table 1). However, the nocturnal nasogastric refeeding group had experienced a significantly greater number of prior hospitalizations (t=–3.41, df=74.0, p<0.05, with Bonferroni correction); the oral refeeding group had experienced 1.3 prior hospitalizations, and the nocturnal nasogastric refeeding group had experienced 2.0 prior hospitalizations on average. Group differences in the occurrence of axis I comorbid conditions, primary amenorrhea, and secondary amenorrhea are displayed in
Table 2.
Table 3 contains unadjusted between-group comparisons of discharge data. The groups did not differ significantly in weight (t=1.31, df=76.5, p=1.00, with Bonferroni correction) or in body mass index (t=2.36, df=94.0, p<0.13, with Bonferroni correction). However, as hypothesized, the nocturnal nasogastric refeeding group differed significantly from the oral refeeding group in weight gain (t=5.01, df=72.4, p<0.05, with Bonferroni correction) and change in body mass index (t=5.16, df=70.9, p<0.05, with Bonferroni correction). Groups did not differ significantly in length of hospital stay (t=0.10, df=91.3, p=1.00, with Bonferroni correction).
The unadjusted results, displayed in
Table 1 and
Table 3, indicate that the oral refeeding patients were admitted for treatment at a slightly higher weight than the nocturnal nasogastric refeeding patients (42.5 kg versus 41.1 kg, respectively), but over approximately the same length of hospital stay, the nocturnal nasogastric refeeding patients experienced a greater than twofold weight gain relative to the oral refeeding patients (5.4 kg versus 2.4 kg), resulting in a higher discharge weight (46.5 kg versus 44.8 kg).
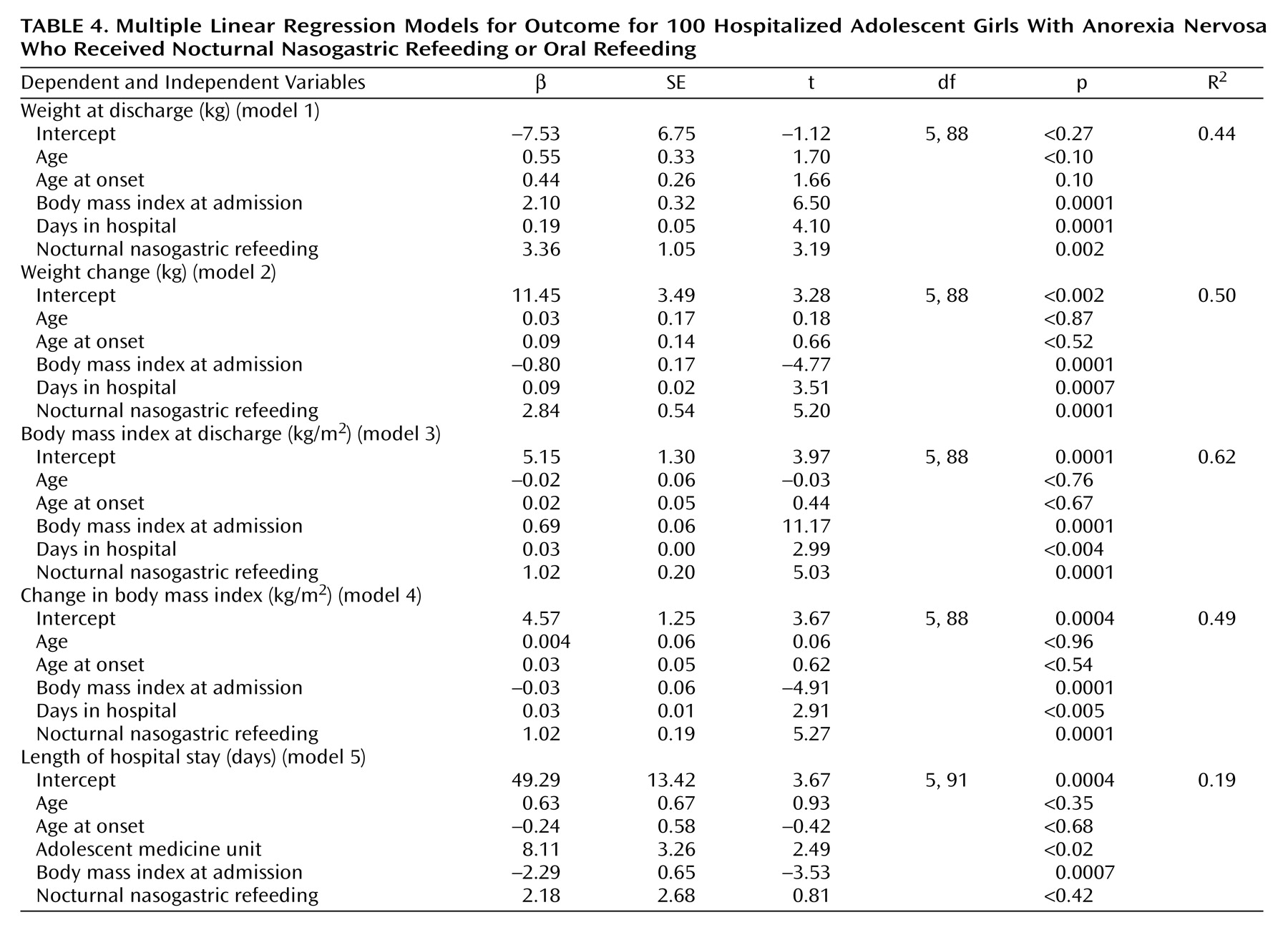
A series of regression models that addressed discharge weight (
Table 3, models 1–4), whether measured in kilograms or body mass index, were consistent in finding that nocturnal nasogastric refeeding was a significant independent predictor of each of these outcomes, relative to standard oral refeeding. Body mass index at admission and length of hospital stay were also significant independent predictors of weight variables at discharge. Neither age at hospital admission nor age at onset of anorexia nervosa were significant predictors of any measures of weight at hospital discharge. Patients treated in the adolescent medicine unit had a significantly longer length of hospital stay than the patients treated in the adolescent psychiatric unit, perhaps attributable to differences in practice patterns that occurred over the course of the study. Regardless of the location of treatment, however, higher body mass index at admission was a significant independent predictor of a shorter hospital stay (
Table 4, model 5).
With regard to nocturnal nasogastric refeeding, adherence complications and side effects were few and relatively minor. Of the 52 patients, two (3.8%) required premedication with a mild antianxiety drug (e.g., 0.5 mg of lorazepam) to relieve anxiety about tube placement. Three patients (5.8%) deliberately removed their nasogastric tubes, requiring tube replacement and consequences similar to those for any infraction of the rules, such as “cheeking” (spitting out) medication (e.g., lack of advancement in the level system of rewards and lack of advancement in eating stage). Side effects were limited to epistaxis in six patients (11.5%) and nasal irritation in 15 patients (28.8%). There were no cases of refeeding syndrome or aspiration pneumonia.
Discussion
Supplemental nocturnal nasogastric refeeding, to complement standard oral refeeding, may be a feasible treatment addition to the nutritional rehabilitation of patients with anorexia nervosa. This study found that supplementation of oral refeeding with nocturnal nasogastric refeeding resulted in significantly greater and more rapid weight gain in a group of hospitalized adolescent females with anorexia nervosa than oral refeeding alone. Patients receiving nocturnal nasogastric refeeding also had a significantly greater change in body mass index than oral refeeding patients. This study is the first of which we are aware to examine the short-term outcomes of nocturnal nasogastric refeeding with a substantial group size. Moreover, this study is the first to compare the relative short-term effectiveness of standard oral refeeding and nocturnal nasogastric refeeding as a supplement to oral refeeding.
A shorter period of hospitalization for the nocturnal nasogastric refeeding group was also hypothesized. This hypothesis was not supported. The oral refeeding and nocturnal nasogastric refeeding groups, in fact, did not differ significantly in their lengths of stay. However, the nocturnal nasogastric refeeding group had experienced approximately 50% more prior hospitalizations than the oral refeeding group. This finding suggests that the nocturnal nasogastric refeeding group may have had a higher degree of pathology and a higher rate of chronicity at admission than the oral refeeding group. Research has demonstrated a negative association between rate of chronicity and amenability to treatment
(3). Thus, this set of findings may support, albeit indirectly, the fundamental position that nocturnal nasogastric refeeding is more effective than oral refeeding in weight restoration during hospitalization. Nocturnal nasogastric refeeding was not only more effective than oral refeeding in weight restoration, but it was also effective in doing so for patients with a higher degree of pathology over the same period of hospitalization.
Because patients in the respective treatment groups began their hospitalizations at different admission weights (41.1 kg for nocturnal nasogastric refeeding subjects and 42.5 kg for oral refeeding subjects), the effect of nocturnal nasogastric refeeding relative to oral refeeding was less apparent in the analyses that did not take into account the patients’ body mass index at hospital admission (
Table 3). The effect of nocturnal nasogastric refeeding relative to oral refeeding is seen more clearly in
Table 4, which adjusts for this critical baseline factor. These results highlight the fact that over similar lengths of hospitalization and despite beginning their treatment at a lower weight or body mass index, the girls treated with nocturnal nasogastric refeeding achieved a greater and more rapid weight gain compared to peers who were provided oral refeeding alone.
Nocturnal nasogastric refeeding appeared also to be comparatively safe in the short term, with few medical or psychological complications attributable to its use. These are important findings given that low discharge weight is implicated in relapse and in light of the current managed health care environment that limits inpatient hospitalizations
(23).
A limitation of this study was the inability to assign subjects randomly to treatment conditions. Thus, certain uncontrolled or unaccounted for factors may have played a role in the outcome of the study. One possible confound may have been treatment setting, which was not entirely equivalent across groups. Specifically, potential disparity in the treatment options of the adolescent medicine unit relative to the adolescent psychiatric unit may have contributed to the resulting observed difference in the effectiveness of nocturnal nasogastric refeeding over oral refeeding. However, additional analysis of the discharge characteristics of the 20 oral refeeding patients treated in the adolescent medicine unit compared with the 28 oral refeeding patients treated in the adolescent psychiatric unit found only one statistically significant difference between the two groups. Patients treated in the adolescent medicine unit had a significantly (t=–4.15, df=41.8, p=0.0002) longer mean length of hospital stay than the patients treated in the adolescent psychiatric unit (27.8 days versus 18.0 days, respectively). This longer hospital stay may reflect the lesser influence of managed care in the early years of the study, when patients were treated in the adolescent medicine unit. There were no statistically significant differences in the admission characteristics of the oral refeeding patients treated in the adolescent medicine unit compared with those in the adolescent psychiatric unit. Finally, there were no statistically significant differences in the effectiveness of nocturnal nasogastric refeeding on the outcome variables in regression analyses that distinguished between oral refeeding patients treated in the adolescent medicine unit and those treated in the adolescent psychiatric unit (data not shown).
Moreover, the effect of treating 28 (58.3%) of the 48 oral refeeding patients on the adolescent psychiatric unit, where all nocturnal nasogastric refeeding subjects were treated, makes the two treatment groups more similar. This similarity across groups would be expected to decrease the probability of finding significant group differences. Nevertheless, significant group differences in change in weight (t=5.01, df=72.4, p<0.05, with Bonferroni correction) and body mass index (t=5.16, df=70.9, p<0.05, with Bonferroni correction) were found, despite the possible presence of this bias. Furthermore, basic practice guidelines were the same across all subjects, with all patients treated by a collaborative team of adolescent medicine physicians and psychiatrists. These factors serve only to strengthen the study’s findings.
Nonetheless, bias may have played a role in the study’s findings. Groups may have varied systematically in some unidentified manner, and those variations, perhaps with regard to patients’ medical and psychosocial histories, may account for the findings. Thus, further research is needed to investigate the short-term and long-term effectiveness of supplemental nocturnal nasogastric feeding in the treatment of patients with anorexia nervosa.