Numerous animal studies have shown that postlearning central or peripheral adrenergic stimulation enhances, while blockade impairs, memory consolidation in a variety of species and tasks
(1). Human studies also indicate that manipulation of adrenergic activity can modulate memory processes
(1–
3). For example, in an earlier study
(2) we found that an arousing story was remembered better than a similar neutral story and that the enhanced memory for the arousing story was blocked by propranolol. Despite this evidence, to our knowledge no demonstration exists that enhanced memory in humans is specifically related to enhanced adrenergic activity during memory consolidation.
In this study we more directly investigated the relationship between enhanced catecholamine activity and memory by conducting a double-blind, placebo-controlled trial using postlearning intravenous infusion of yohimbine to stimulate norepinephrine during consolidation of memory for the same emotionally arousing story that was used in our propranolol study
(2). We hypothesized that subjects given yohimbine would have a significantly higher mean memory score for the story than subjects given placebo and that long-term memory for the story would be positively correlated with levels of plasma 3-methoxy-4-hydroxyphenylglycol (MHPG), a major metabolite of norepinephrine.
Method
Twenty-one healthy men and nine healthy women between the ages of 20 and 54 (mean age=32.4 years, SD=10.9) gave written informed consent and were free of major medical illnesses and free of psychopathology as determined by the Structured Clinical Interview for DSM-III-R. On the first test day, subjects viewed 12 slides that depicted an emotionally arousing short story. These same slides have been used in adrenergic blockade studies
(2,
3) and are described in detail elsewhere
(2). Five minutes after viewing the slides, subjects received either intravenous yohimbine (0.4 mg/kg, N=14) or intravenous placebo (0.9 NaCl, N=16) in double-blind randomized fashion. Blood samples for plasma free MHPG were drawn at 30 and 15 minutes before administration of yohimbine or placebo and 20, 60, and 120 minutes after.
The subjects returned 1 week later for a second day of testing. To control for nonspecific stress effects, subjects had an intravenous line inserted at this time. One hour later, subjects took a surprise memory test for the slides they had viewed on the first day.
Plasma concentration of MHPG, a marker of central and peripheral norepinephrine activation, was determined by gas chromatography–mass spectrometry according to a slightly modified version of the method of Elsworth et al.
(4). Analyses were performed with within-day coefficients of variation of less than 5%; day-to-day coefficients of variation for low-level (2.19 ng/ml) and high-level (7.56 ng/ml) quality assessment sample pools were 13.5% and 8.5%, respectively. Data on MHPG levels were not available for one of the patients who received placebo.
Results
Peak change in MHPG was significantly greater for the yohimbine group (mean=1.48 ng/ml, SD=0.93) than for the placebo group (mean=0.63 ng/ml, SD=0.37) (t=3.21, df=26.8, p=0.005, unequal variances). The range of MHPG change values in the yohimbine group (0.22 to 3.27 ng/ml) was also greater than the range in the placebo group (–0.02 to 1.11 ng/ml). Seven of the subjects who received yohimbine had a peak change in MHPG (range=0.22 to 1.10 ng/ml) that was no greater than the peak change observed for each of the 15 subjects who received placebo. That is, for these seven subjects, yohimbine was no more effective than placebo in releasing plasma norepinephrine and MHPG.
Memory recall did not differ significantly between the yohimbine group (mean=0.58, SD=0.06) and the placebo group (mean=0.56, SD=0.09) (t=1.01, df=25.1, p=0.32, unequal variances). However, the seven yohimbine subjects with the highest peak increases in MHPG (peak changes in MHPG that were greater than those observed in all 15 placebo subjects) had a significantly higher mean memory score than the seven yohimbine subjects with the lowest peak increases in MHPG (peak changes in MHPG that were no greater than those observed in any of the 15 placebo subjects) (t=2.22, df=12, p<0.05).
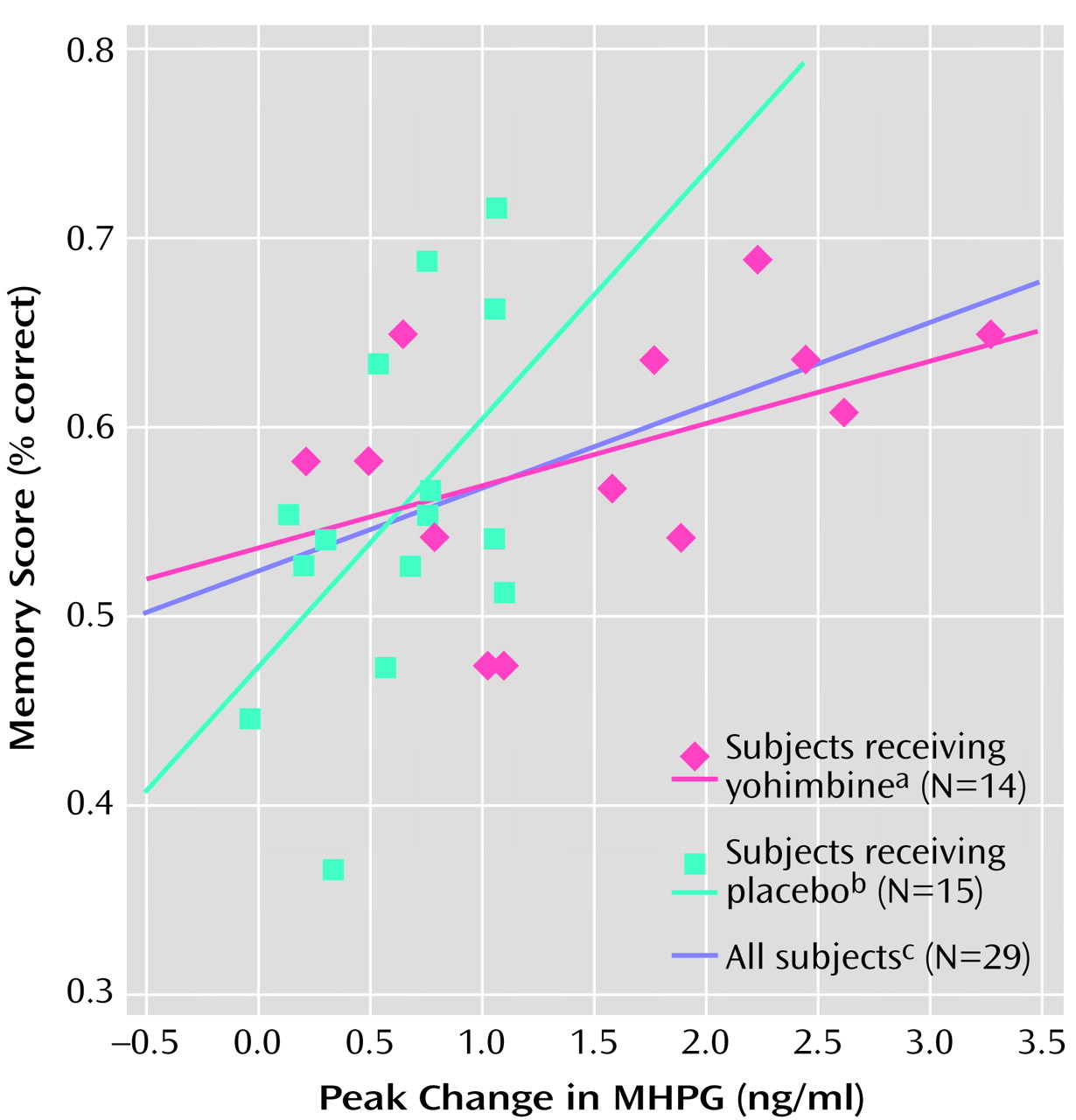
Linear regression with a dummy variable for group (0, 1) was used to estimate the relationship between MHPG and memory by group. The effect of MHPG increase on memory was not significantly different between subjects in the yohimbine and placebo groups (p=0.09) (
Figure 1). For the group as a whole (yohimbine subjects plus placebo subjects) there was a significant positive correlation between peak change (from baseline) in MHPG and memory for the story (r=0.44, R
2=0.19, N=29, p<0.02). The correlation between peak change in MHPG and memory for the story was also significant for the placebo group alone (r=0.52, R
2=0.27, N=15, p<0.05) but fell short of significance for the yohimbine group alone (r=0.47, R
2=0.22, N=14, p=0.09) (
Figure 1). Although the slope of the placebo group was steeper than the slope of the yohimbine group, the two slopes did not differ significantly.
Discussion
These findings support the hypothesis that enhanced noradrenergic activity during memory consolidation is associated with enhanced long-term memory in humans. For the group as a whole and for the placebo group alone there was a significant positive correlation between peak change in plasma MHPG at the time of memory consolidation and memory for a series of slides depicting an emotionally arousing story. Although the yohimbine group did not have higher memory scores than the placebo group, in half of the subjects who received yohimbine, it was no more effective than placebo in activating the norepinephrine system (as evidenced by MHPG levels). Since the hypotheses in this study predicted that elevated norepinephrine activity during memory consolidation would enhance memory storage, there is no reason to expect that memory would be enhanced more in yohimbine-infused subjects who lacked an adequate MHPG response than in subjects given placebo. Indeed, the seven yohimbine subjects with enhanced norepinephrine activity (peak MHPG changes greater than those observed in all placebo subjects) had a clear enhancement of memory compared with the seven yohimbine subjects without elevated peak MHPG responses (peak MHPG changes no greater than those observed in placebo subjects).
A major strength of this study is that yohimbine was administered
after learning. Thus, the norepinephrine-related improvements in memory could not have resulted from effects on attentional or motivational processes occurring during encoding and must instead have related to effects on postlearning consolidation processes. It is not clear why yohimbine failed to increase MHPG above placebo levels in seven subjects, but this finding is consistent with those of other studies demonstrating significant intersubject variability in plasma norepinephrine and MHPG responses to yohimbine
(5). Irrespective of the factors accounting for this variability in response to yohimbine, the present findings remain the first to our knowledge to indicate that enhanced norepinephrine during memory consolidation results in enhanced long-term memory in humans.
An important issue for future work concerns potential interactions between postlearning stimulation and degree of endogenous norepinephrine activation produced by the to-be-remembered stimuli at encoding
(6). Future studies should consider the use of both neutral and traumatic stories, crossover designs in which subjects view two separate stories (one followed by yohimbine and one followed by placebo), and inclusion of other neuromodulators known to affect memory consolidation, such as epinephrine and glucose.
The present study suggests that enhanced memory in humans is associated with elevated norepinephrine activity during memory consolidation. It has been proposed that enhanced memory for arousing events has significance for survival and that catecholamine-mediated enhancement of memory consolidation for arousing and traumatic events may play a role in the reexperiencing symptoms of posttraumatic stress disorder
(7).