Several studies have examined the contributions of genetic and environmental factors to hippocampal volumes in patients with schizophrenia by using structural magnetic resonance imaging (MRI). Genetic etiological influences are suggested by findings of higher intraclass correlations in discordant monozygotic than in dizygotic twins
(1) and smaller volumes relative to healthy comparison subjects in both adolescents at genetic risk for schizophrenia
(2–
4) and in unaffected siblings of schizophrenic patients
(5,
6). A role for nongenetic etiological influences is suggested by findings of smaller volumes in the affected compared with the unaffected co-twin of monozygotic twins discordant for schizophrenia
(7,
8).
While predisposing genes have yet to be identified, obstetric complications, in particular those associated with fetal hypoxia, are among the strongest environmental risk factors for schizophrenia
(9). To date, two MRI studies have examined the relationship of obstetric complications with hippocampal volumes in patients with schizophrenia. Stefanis and colleagues
(10) found smaller hippocampal volumes in schizophrenic patients with a history of pregnancy and birth complications but not in patients from multiply affected families. McNeil and colleagues
(8) found that the intrapair differences in rostral hippocampal volumes between monozygotic twins discordant for schizophrenia were related to higher rates of obstetric complications. While these results are encouraging, obstetric complications were assessed retrospectively in both studies through maternal interview, which is not considered as reliable as prospective assessment
(11,
12).
One question that has yet to be addressed is whether genetic and obstetric influences on hippocampal volumes in schizophrenia are independent, additive, or interactive. No prior study has had access to hippocampal volume data on subjects at multiple levels of genetic predisposition for schizophrenia and data on obstetric complications within the same sample. Mednick
(13) was the first to suggest a possible interaction between predisposing genes and fetal hypoxia in the etiology of schizophrenia, specifically implicating the hippocampus. More recently, Lipska and Weinberger
(14) showed that spontaneous and amphetamine-induced behavioral effects of neonatal hippocampal lesions in rats (hyperlocomotion) were specific to strain and lesion size, suggesting that the degree of genetic predisposition and the extent of neonatally induced hippocampal lesions contribute to a pattern of behavioral outcome in adolescent rats that mimics that of schizophrenic patients.
There is growing evidence that fetal hypoxia predicts early-onset schizophrenia
(15–
17) and that adolescent-onset schizophrenic patients perform worse than adult-onset patients on cognitive functions involving the hippocampus, such as remote memory
(18) and recent memory and executive functions
(19). Several postmortem and MRI studies have reported positive correlations between hippocampal volume and age at onset
(10,
20,
21).
On the basis of these findings, our first hypothesis was that consistent with genetic influences on hippocampal volumes, the hippocampal volumes of probands would be smaller than those of their full siblings, which in turn would be smaller than those of healthy comparison subjects. Our second hypothesis was that genetic predisposition and a history of fetal hypoxia would interact in predicting hippocampal volume, i.e., fetal hypoxia would have a larger effect in a group at high genetic risk for schizophrenia than in those at low genetic risk. Third, consistent with this role of nongenetic influences on hippocampal volumes, we hypothesized that the intraclass correlations of discordant sibling pairs would be smaller than those of healthy sibling pairs. Our final hypothesis was that hippocampal volumes in patients would relate positively to age at onset.
Method
Subject Ascertainment
The participants were selected by searching the Finnish Population Register for all individuals born in Helsinki in 1955 (N=7,840) and all of their first-degree relatives (N=26,273, consisting of 12,796 siblings and 13,477 parents). This cohort was screened for a history of psychiatric treatment through national computerized databases that used methods previously described
(22,
23), and potential probands were selected at random from this pool. Eligibility was restricted to probands with a lifetime DSM-III-R diagnosis of schizophrenia or schizoaffective disorder, two disorders with known common familial predisposition
(24). Approximately 75% (80 probands) of those approached provided written informed consent and met inclusion criteria. An attempt was made to recruit at least one nonschizophrenic sibling of each studied proband, but this was possible for only 62 of the 80 cases. Healthy comparison subjects (N=28 sibling pairs) were chosen from the same birth cohort to match probands and their siblings on demographic variables; those with a personal or family history of psychiatric treatment were excluded. High-resolution MRI scans were obtained for 75 patients, 60 siblings, and 53 healthy comparison subjects. Technical problems with the MRI scans excluded five subjects (three probands, two siblings) from group analysis, leaving 72 probands (60 with schizophrenia and 12 with schizoaffective disorder), 58 siblings, and 53 healthy comparison subjects from which were formed 60 illness-discordant and 25 unaffected sibling pairs from 45 and 25 independent families, respectively.
Diagnostic Evaluation
All subjects were interviewed by using the Structured Clinical Interview for DSM-III-R (SCID), patient and nonpatient editions
(25). Siblings and healthy comparison subjects were also interviewed and rated on the cluster A items from the Personality Disorders Examination
(26). Diagnostic reliability was excellent (mean kappa=0.94, SD=0.02)
(27). Final diagnoses were made by consensus among three independent raters. Age at onset was defined according to SCID criteria as the age at first psychotic symptoms
(25). The groups of patients, siblings, and healthy comparison subjects were balanced in terms of major demographic variables (
Table 1).
Obstetric Records
A standard form was used to code information on maternal health, fetal monitoring, prenatal and perinatal complications, and neonatal conditions from the original antenatal clinic and obstetric hospital records by a worker blind to diagnosis and imaging results. Fetal hypoxia was scored as present if the subject was coded as blue at birth or neonatally or had two or more complications that were significantly related to birth or neonatal asphyxia in the overall sample: umbilical cord knotted or wrapped tightly around neck, placental infarcts, third trimester bleeding, preeclampsia, maternal anemia or anorexia during pregnancy, fetal heart rate/rhythm deviations, breech presentation, and premature (≥2 weeks) birth. Details of the scale derivation and its validation in predicting early-onset schizophrenia have previously been established
(16).
Imaging Procedures
Acquisition
MRIs were acquired on a 1.5-T scanner (Siemens Medical Systems, Iselin, N.J.) in the Department of Radiology, University of Helsinki by using a standard MPRAGE sequence (TR=10 msec, TE=4 msec, flip angle=12°, and no interslice gap). The matrix size was 256×256×128 pixels, corresponding to a field of view of 25 cm2 and a resolution of 0.98×0.98×1.3 mm.
Segmentation and reslicing
After deleting nonbrain voxels
(23), images were segmented into gray matter, white matter, and CSF by using an adaptive, three-dimensional, Bayesian algorithm
(29), previously validated for this purpose
(30). In order to control for differences in head tilt during acquisition, images were resliced parallel to the anterior commissure-posterior commissure plane by using methods previously described
(23) and saved in sagittal and coronal views.
Anatomical tracings
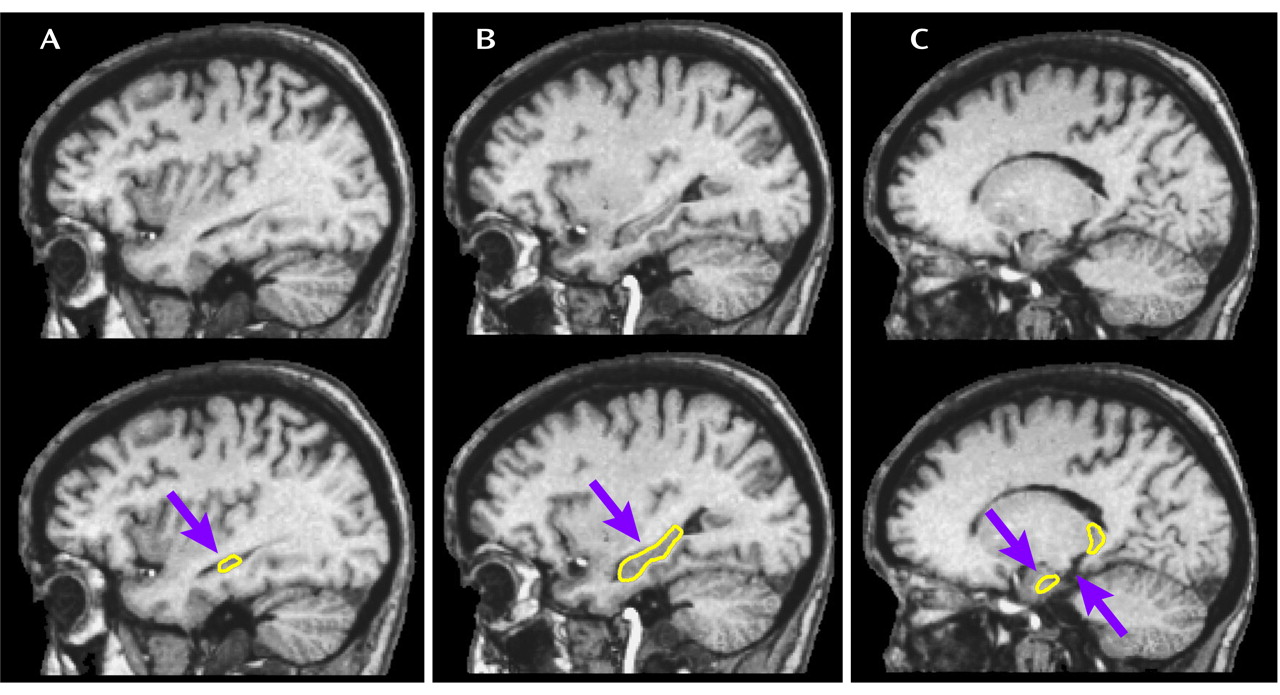
The tracing method was developed by two of the authors (P.A.S. and T.G.M.v.E.) and is depicted in
Figure 1. Hippocampi were outlined in the sagittal and coronal views, and the drawings were projected onto their coronal and sagittal views, respectively, in order to examine coherence. On the basis of these projections, a protocol was established for tracing the hippocampus in the sagittal view. Dr. Arnold B. Scheibel, an eminent neuroanatomist at the UCLA Brain Research Institute with specific expertise in hippocampal anatomic abnormalities in schizophrenia
(31), examined the protocol and approved the delineation. The hippocampal volume measures included the cornu Ammonis, the gyrus dentatus, the presubiculum, and the subiculum proper. Tracings were performed blind to diagnosis, birth history, hemisphere, and orientation (neurological-radiological). Volume counts included only gray matter voxels in the region of interest. Interrater and intrarater reliabilities based on 10 cases were excellent (>0.95).
Statistical Analyses
The data were analyzed by using the general linear mixed model with repeated measures (SAS 6.12 [SAS Institute, Cary, N.C.]). We corrected for dependency (i.e., correlation) among multiple observations from the same family (i.e., siblings) by treating family as a random variable
(32) and adjusting the model error terms accordingly. Degrees of freedom were estimated from the data by using the Satterthwaite option. We tested the hypothesis that genetic risk for schizophrenia would be associated with smaller hippocampal volume by modeling risk group (proband, sibling, healthy comparison) as a fixed-effect predictor while covarying for age at examination, history of substance disorder, gender, the interaction of group with history of substance disorder and with gender, and total brain volume
(33). To test for possible differences in overall or between-group laterality, hemisphere and group-by-hemisphere were entered into the model as a within-subject repeated-measures factor and an interaction term, respectively. Significant main effects were followed up with one-tailed t tests.
We tested the hypothesis that a history of fetal hypoxia would be more strongly associated with smaller hippocampal volume in the presence of genetic susceptibility to schizophrenia by modeling hypoxia group, risk group (probands, siblings, and healthy comparison subjects as well as probands versus nonprobands) and the risk group-by-hypoxia group interaction as fixed-effect predictors, followed by planned one-tailed t tests that compared subjects with and without a history of fetal hypoxia by risk group. Age at scan, gender, history of substance disorder, and total brain volume were entered as covariates. The significance of each predictor was tested while accounting for all other model terms simultaneously.
Intraclass correlations (ICCs) and their confidence intervals for sibling pairs discordant for schizophrenia or schizoaffective disorder and unaffected sibling pairs were calculated and compared by using one-tailed t tests. The relationship with age at onset was examined by using a mixed-model regression analysis with age at onset as a continuous predictor and gender, age at scan, substance disorder, total brain volume, and duration of illness entered as covariates, followed by a one-tailed t test on the slope estimate.
Results
Group Differences
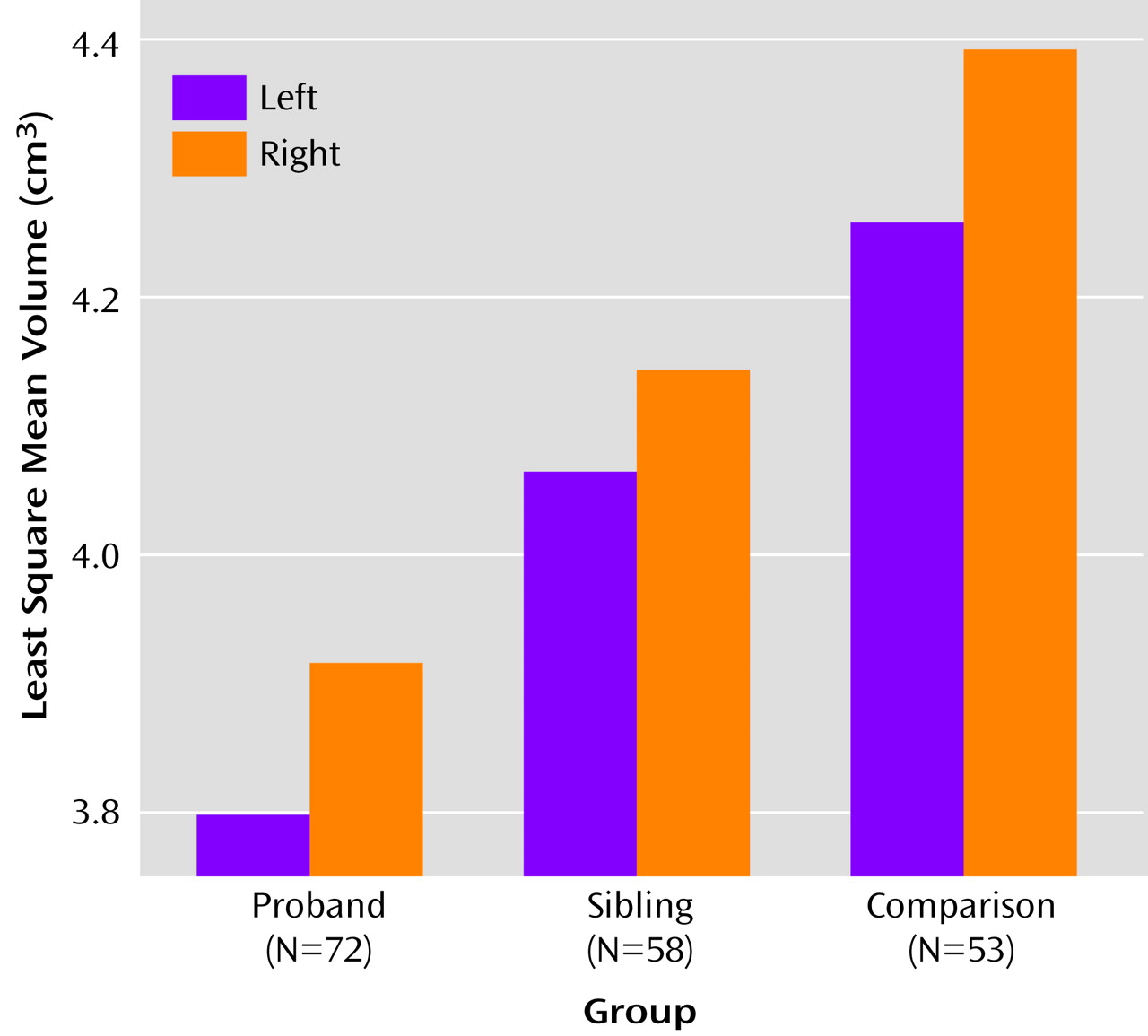
As seen in
Figure 2, the group-wise analysis showed significant effects for risk group (F=9.49, df=2, 117, p=0.0002) and hemisphere (F=24.06, df=1, 177, p=0.0001), but there were no significant interactions of risk group with hemisphere, gender, or substance abuse in predicting hippocampal volume. Apart from total brain volume (F=17.34, df=1, 105, p=0.0001), none of the covariates significantly predicted left or right hippocampal volumes. Apart from gender, which showed a significant main effect (F=8.50, df=1, 108, p<0.005) driven by larger hippocampi in men than in women, none of the model terms changed when analyses were run excluding total brain volume as a covariate.
On the basis of the foregoing, contrast analyses were performed on total hippocampal volumes, modeling the data without the nonsignificant interaction terms. The total hippocampal volume of the probands with schizophrenia or schizoaffective disorder was significantly smaller than that of their healthy full siblings (t=2.17, df=117, p<0.02) and healthy subjects (t=5.24, df=117, p=0.00005), and the total hippocampal volume of the unaffected siblings was significantly smaller than that of healthy comparison subjects (t=3.15, df=118, p=0.001).
Relationship With Fetal Hypoxia
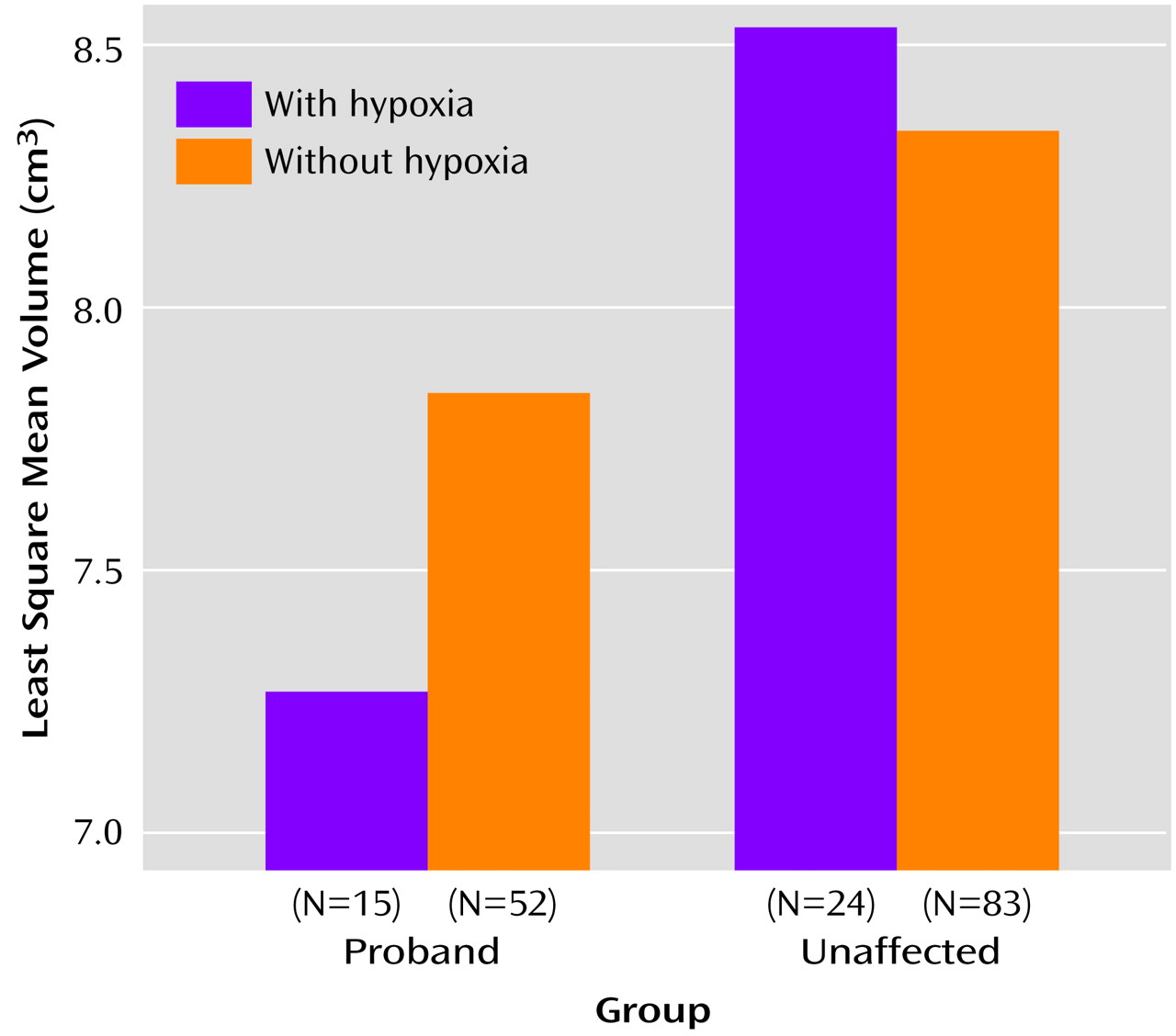
The analysis examining the relationship with hypoxia did not show a significant main effect for hypoxia (F=0.20, df=1, 156, p=0.66) nor a significant group-by-hypoxia interaction (F=1.91, df=2, 145, p=0.15). However, the planned contrasts did show a significant effect for hypoxia in the predicted direction within the proband group (t=1.88, df=155, p=0.03), with smaller hippocampal volume seen in the patients exposed compared with those not exposed to fetal hypoxia. This pattern suggests that hypoxia may have only influenced the probands’ hippocampal volumes and that the analyses lacked the power to detect this. Subsequent analyses to increase power, comparing probands with and without hypoxia to healthy subjects (unaffected siblings and comparison subjects combined) with and without hypoxia (depicted in
Figure 3), showed a marginally significant group-by-hypoxia interaction (F=3.79, df=1, 138, p=0.054). Contrast analyses indicated that this interaction was driven by a small effect of hypoxia in the proband group (t=1.81, df=154, p<0.04, effect size=0.23) but not in the combined group of unaffected siblings and healthy comparison subjects (t=–0.72, df=151, p=0.24).
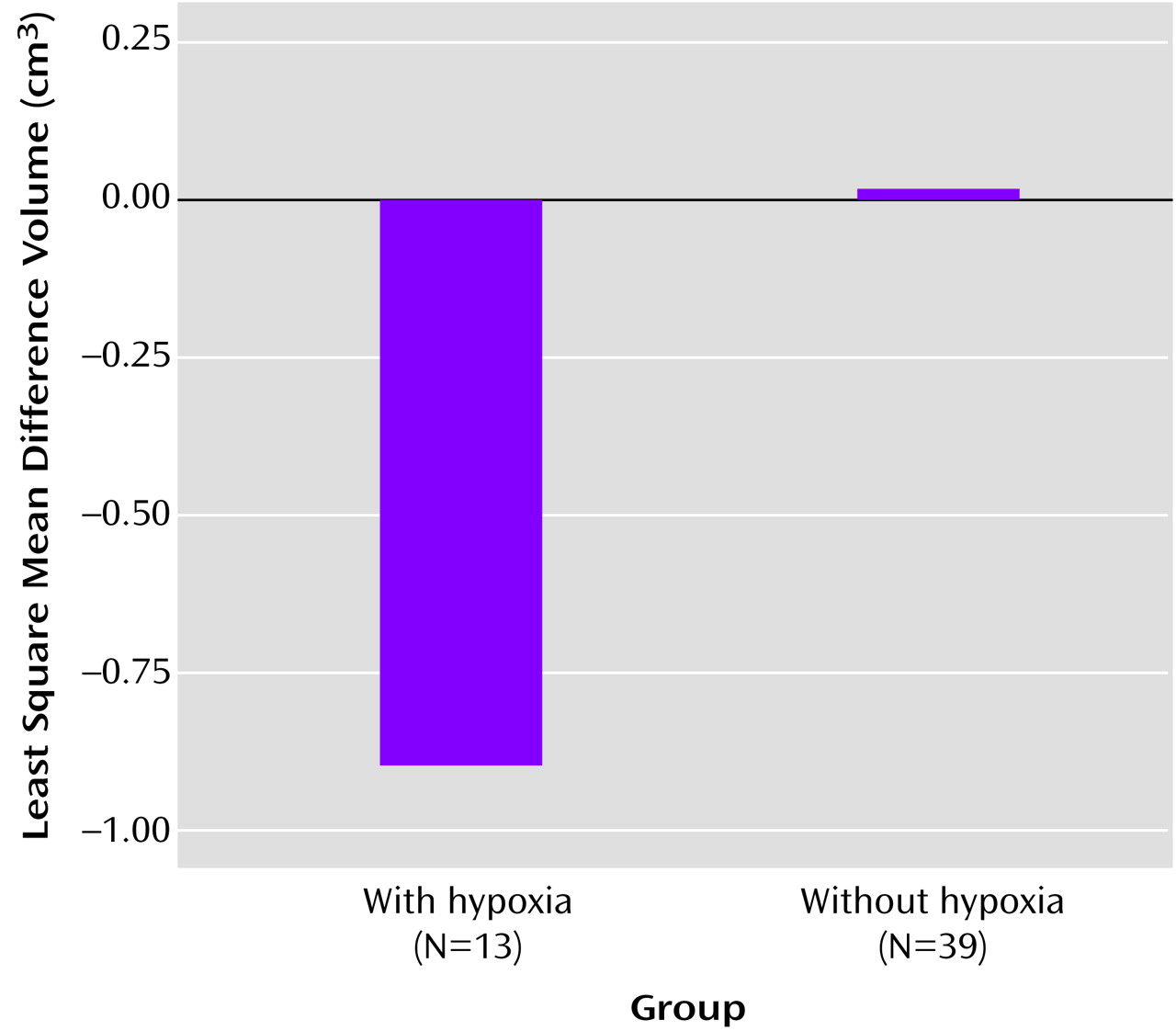
Additional analyses to corroborate these findings that compared the relation of fetal hypoxia to hippocampal volume differences between the probands and their unaffected siblings showed a marginally significant main effect for hypoxia group (F=3.36, df=1, 46, p=0.07) independent of age, gender, and total brain volume (
Figure 4). Also, the mean difference volume for the patients with hypoxia was significantly smaller than 0 (t=–2.11, df=46, p=0.02), while the mean difference volume for patients without hypoxia was not (t=0.06, df=46, p=0.48).
Intraclass Correlations
One-tailed t tests that compared correlations in hippocampal volume (raw and after correction for total brain volume) between sibling pairs discordant for schizophrenia or schizoaffective disorder and unaffected sibling pairs showed that the healthy sibling pairs had significantly higher ICCs than did the illness-discordant pairs (
Table 2).
Relationship With Onset
Age at onset significantly predicted total hippocampal volume (t=1.78, df=63, p=0.03; slope=0.05, SE=0.027) independently of gender, age at scan, substance disorder, total brain volume, and duration of illness. These results indicate that the probands with smaller hippocampal volumes were likely to have experienced an earlier onset of schizophrenia or schizoaffective disorder than those probands with larger hippocampal volumes.
Discussion
The principal findings of this study were that 1) hippocampal volume differences occurred in a stepwise fashion with each increase in genetic predisposition to schizophrenia, and 2) the hippocampal volumes of probands exposed to fetal hypoxia were smaller than of those unexposed, while no such relationship was observed within the healthy groups (i.e., unaffected siblings or unrelated comparison subjects).
We interpret the stepwise decrease in hippocampal volumes with increased genetic predisposition to suggest that disease genes contribute to smaller hippocampal volume reductions in patients with schizophrenia or schizoaffective disorder. Shared environmental factors are less likely to account for a substantial amount of the variance in hippocampal volumes, since twin and adoption studies have shown that shared genes and unique environmental factors, rather than shared biological or familial environments, underlie the higher risk for schizophrenia
(22). Apart from one study that reported a null finding
(34), our findings are consistent with previous studies that show smaller hippocampal volumes in genetically predisposed individuals relative to comparison subjects
(1–
6).
We specifically examined the relationship between fetal hypoxia and hippocampal volume at multiple levels of genetic predisposition in an attempt to dissociate models on the mode of action of genes and fetal hypoxia in patients with schizophrenia or schizoaffective disorder. We interpret our finding of smaller hippocampal volumes in probands with fetal hypoxia than in probands without fetal hypoxia (whereas no such relationship was evident within siblings or comparison subjects) as evidence for the interaction model. This is consistent with our previous report of a significant relationship between fetal hypoxia and ventricular volumes in the patients only
(35) and may suggest that this vulnerability is polygenic in origin and, as such, less likely to occur in unaffected siblings who share on average 50% of the disease genes.
It is important to note that the frequency of hypoxia-related insults was the same across all three groups, which rules out the gene-environment covariation model in which an increase in genetic predisposition would predict an increase in the number of hypoxic events. Since it is improbable that the hypoxic events in the probands were of a larger magnitude than the hypoxic events in the unaffected siblings and comparison subjects, the additive model is a less likely candidate also. The current results corroborate earlier findings relating a history of fetal hypoxia to brain volumes in schizophrenia
(8,
10,
35).
The ICCs for the healthy sibling pairs suggest that hippocampal volumes are largely determined by genetic factors in the general population, while the ICCs for the discordant pairs suggest either larger variation in genes or unique environmental factors that influence hippocampal volumes independently of those that influence total brain volume. Consistent with recent high-resolution MRI studies
(36–
38) and reviews
(39,
40), we found smaller left than right hippocampal volumes in all groups.
Our results favor the two-hit model in that an early event (fetal hypoxia) has influence on an anatomical region of the brain, the volume of which is linked to the onset of schizophrenia. The results encourage future studies examining the relationship between fetal hypoxia and brain abnormalities in schizophrenia and the search for genes that increase vulnerability to fetal hypoxia.
Strengths of the study include the use of a random representative population sample so that the results could be generalized to the population; availability of probands’ relatives and comparison subjects so that competing etiologic models of hippocampal abnormalities could be compared; availability of discordant and healthy sibling pairs so that intraclass correlations could be compared directly; prospective gathering of pregnancy and birth complications from the original pregnancy and birth records; use of high-resolution images to make the measurements; high reliabilities achieved on the measurements; and the blind presentation of the images (neurological/radiological) during data collection so that potential rater or other orientation biases were eliminated in examination of the hemispheric asymmetries.
Several weaknesses of the study must also be noted. Since the data are correlational in nature, we cannot exclude the possibility that a confounding factor relating to onset may be responsible for the observed relationship between fetal hypoxia and smaller hippocampal volume. Unlike the cortical findings
(35), the relationship between hypoxia and hippocampal volume differences between probands and their unaffected siblings did not remain significant after covarying for age at onset (F=1.07, df=1, 44, p=0.30). However, this type of analysis runs the risk of “throwing away the baby with the bath water,” especially since hippocampal volumes also predict age at onset. Second, the measurements only reflect hippocampal volumes, and it is possible that there are also regional shape changes, in particular in the Sommer’s sector. Third, the relationship of fetal hypoxia and hippocampal volume was examined on average 40 years after the presumed hypoxic insults took place. A more sensitive approach may be to examine the relationship of fetal hypoxia and hippocampal volume in at-risk neonates, children, or adolescents, since other processes that influence hippocampal volumes, such as stress and synaptic pruning, may not have had time yet to exert such a large influence.