In an effort to better understand how new medications diffuse into use, we looked at prescription patterns for antipsychotic medications in the VA health care system. Using prescription drug data, we determined 1) the proportion of patients who had different stable antipsychotic regimens who were subsequently switched to another drug, 2) how much time elapsed before they were switched, 3) the specific drugs to which they were switched, and 4) whether they stayed on the new drug regimen or were switched back to the former drug. We thus sought to determine whether the diffusion of new medications occurs by progressive replacement of older agents or by a more dynamic process of switching across a variety of agents.
Results
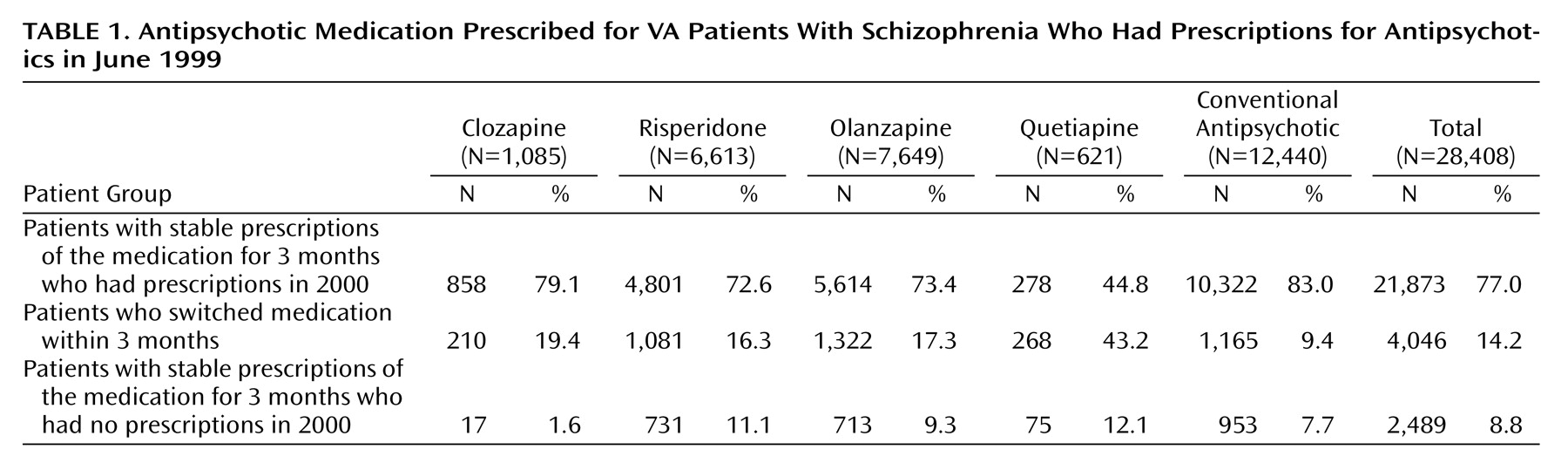
Table 1 describes how the initial sample of patients who had stable antipsychotic regimens was constructed. Of a total of 28,408 patients with a diagnosis of schizophrenia who had a prescription for an antipsychotic in June 1999, 77% remained on the drug for 3 months and had a prescription in the following year (fiscal year 2000), 14% switched medications within 3 months, and the remainder (9%) had stable prescriptions for 3 months but did not have a prescription drug record in the next year. A total of 5,426 patients were switched to a new medication during fiscal year 2000.
The most frequently prescribed medications in the initial sample were conventional antipsychotics (12,440 patients [43.8%]), followed by olanzapine (7,649 patients [26.9%]), and risperidone (6,613 patients [23.3%]). Few patients were prescribed clozapine (1,085 patients [3.8%]) or quetiapine (621 patients [2.2%]). Stability (i.e., not switching medications) was most common for patients prescribed conventional antipsychotics and clozapine (83.0% and 79.1%, respectively) and was least common for patients prescribed quetiapine (44.8%).
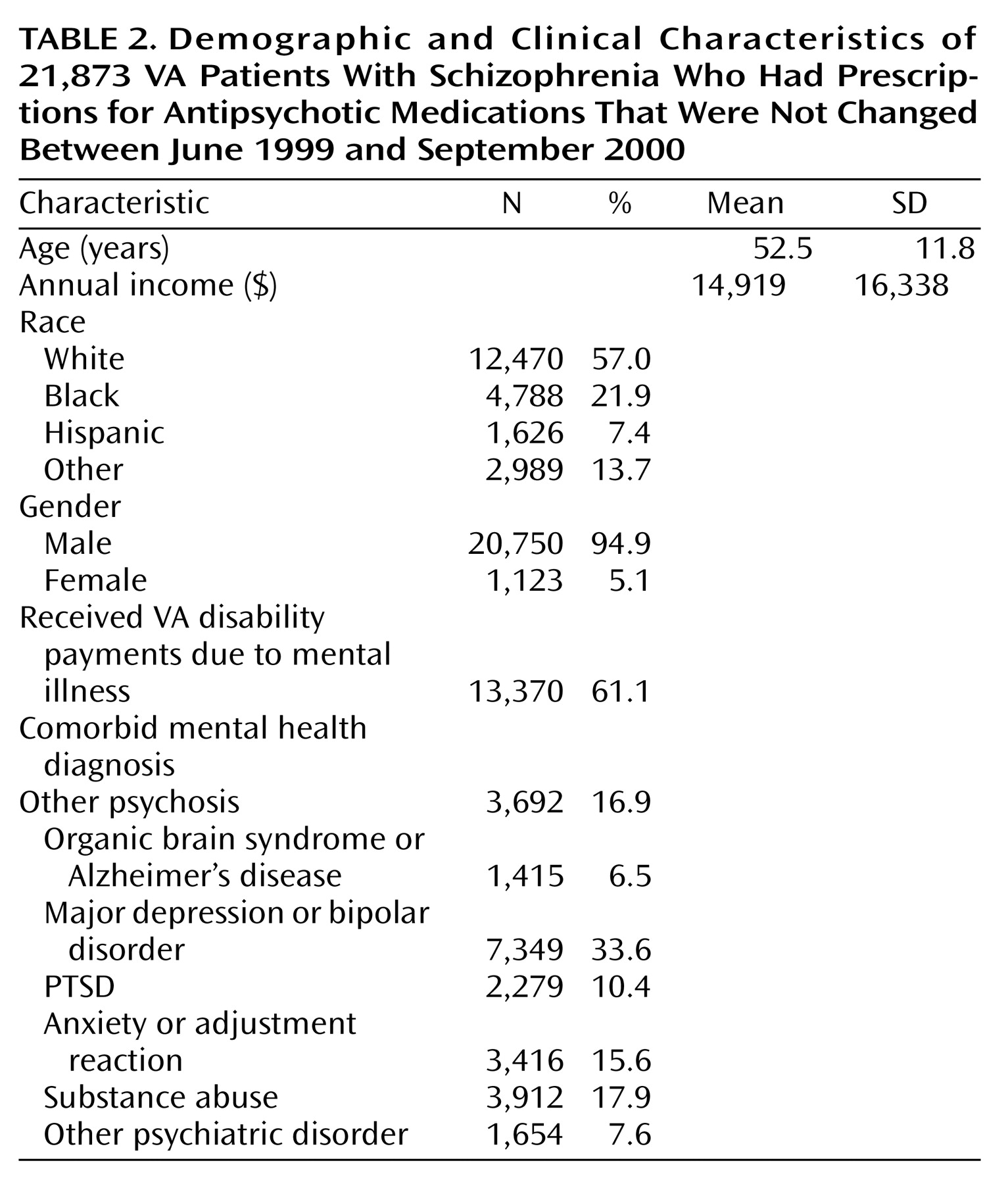
Table 2 shows some characteristics of this stable group. The sample is overwhelmingly male (95%), which is characteristic of the VA population, and had an average age of 52.5 years. The most common comorbid diagnoses were major depression or bipolar disorder and substance abuse. Organic brain disorder or Alzheimer’s disease and other psychiatric disorder were the least common comorbid diagnoses.
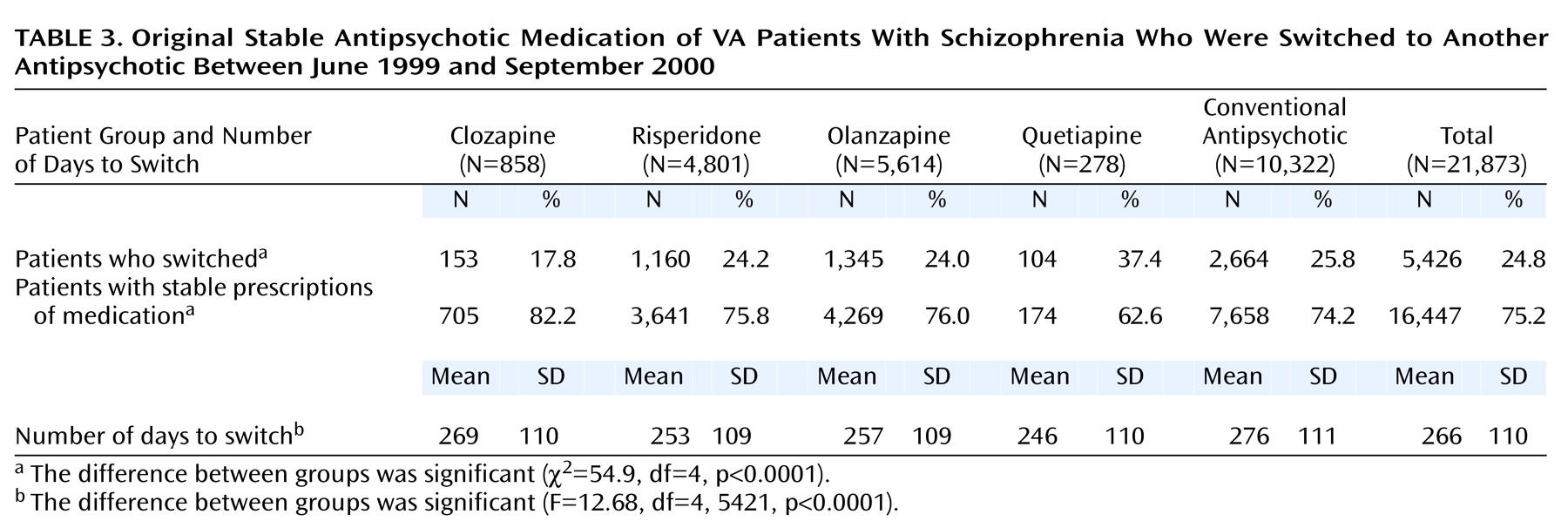
Table 3 shows the number of patients with stable regimens who subsequently switched medications and the mean length of time on the original drug. Results are presented by the original stable therapy. As in the initial study sample, patients who received stable prescriptions of quetiapine were most likely to switch and switched sooner than patients with stable prescriptions of other medications. Patients with stable prescriptions of clozapine were the least likely to switch, whereas those with stable prescriptions of conventional antipsychotics stayed on their medication the longest before switching. The differences between these groups in the proportion that switched drug therapy are statistically significant, as are the differences in the length of time on the original therapy (
Table 3).
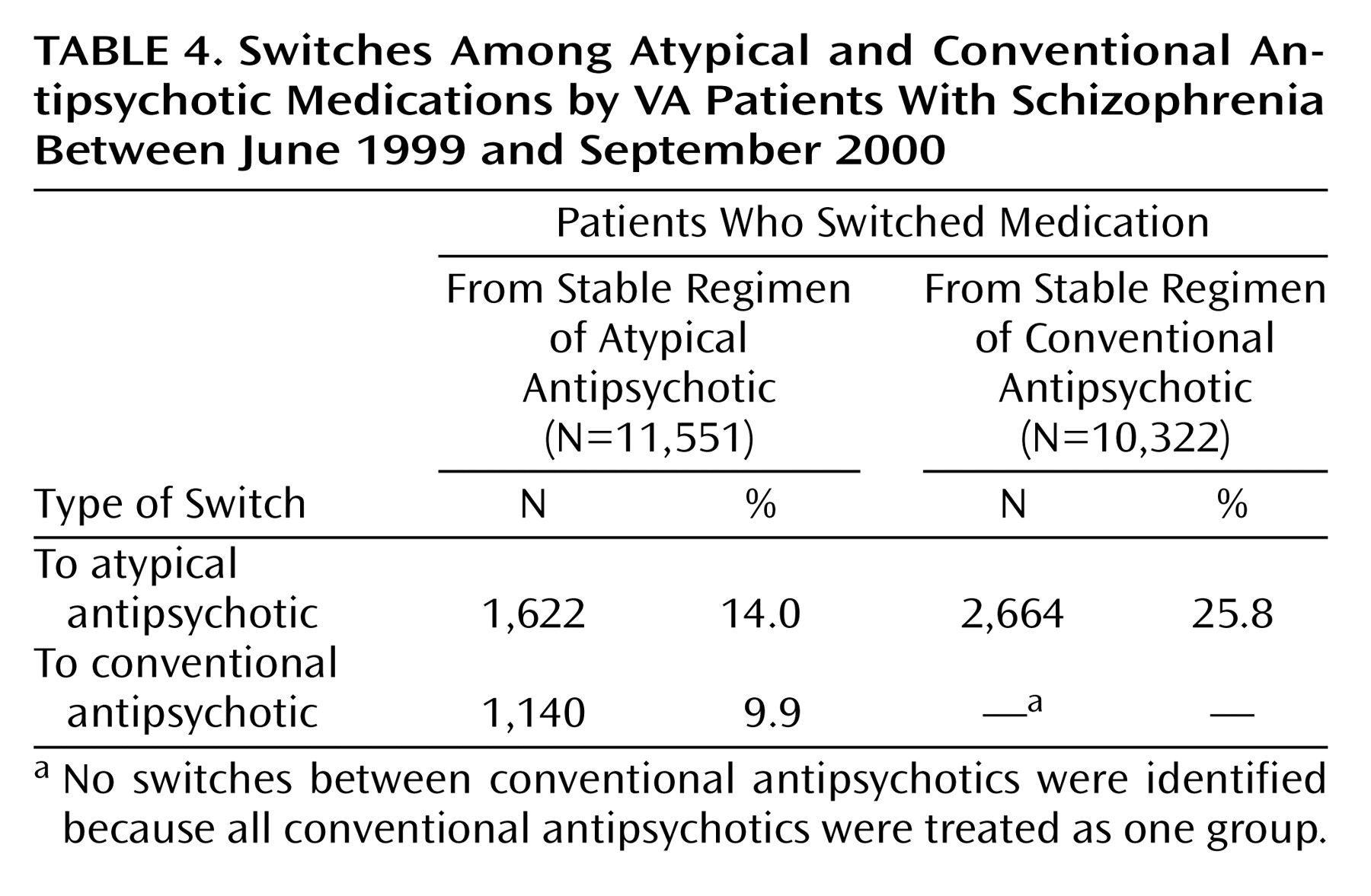
Table 4 summarizes the switching patterns by class of medication. The most common switching pattern was from a conventional to an atypical medication, followed by switching from one atypical drug to another and switching from an atypical to a conventional medication.
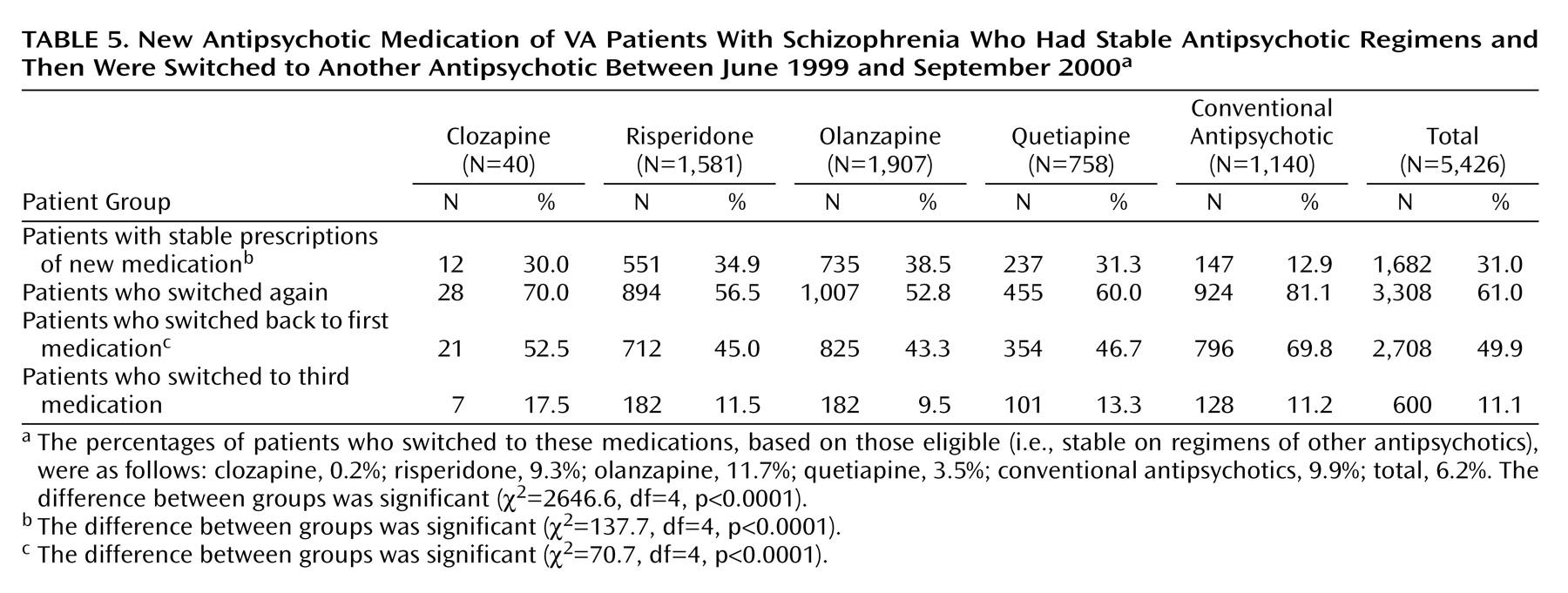
Table 5 reports the specific medications to which patients switched. A total of 5,426 patients switched to a new medication during fiscal year 2000. The greatest number of patients (1,907 [35.1%]) switched to olanzapine, followed by risperidone (1,581 [29.1%]), conventional antipsychotics (1,140 [21.0%]), quetiapine (758 [14.0%]), and clozapine (40 [0.7%]). Half of these patients eventually switched back to their original drug by the end of the fiscal year (
Table 5). Patients who switched to a conventional antipsychotic were more likely to switch back to their original medication than patients who switched to an atypical medication (
Table 5). Only 31% of patients who switched maintained a stable regimen on their new medication.
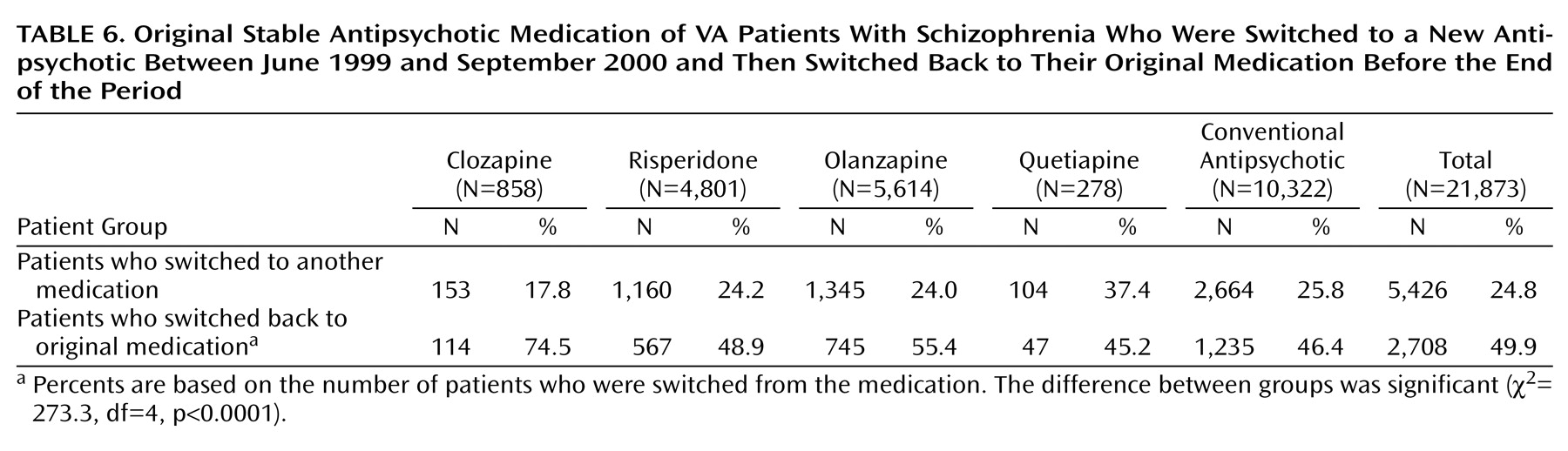
Table 6 reports more information for patients who switched back to their original stable medication. Patients whose original stable medication was clozapine or olanzapine who switched medications were most likely to switch back to their original therapy by the end of the year. Patients whose original stable medication was quetiapine who switched to a new medication were the least likely to switch back to their original drug, followed closely by those whose stable mediation was a conventional antipsychotic. These differences were statistically significant (
Table 6).
Finally,
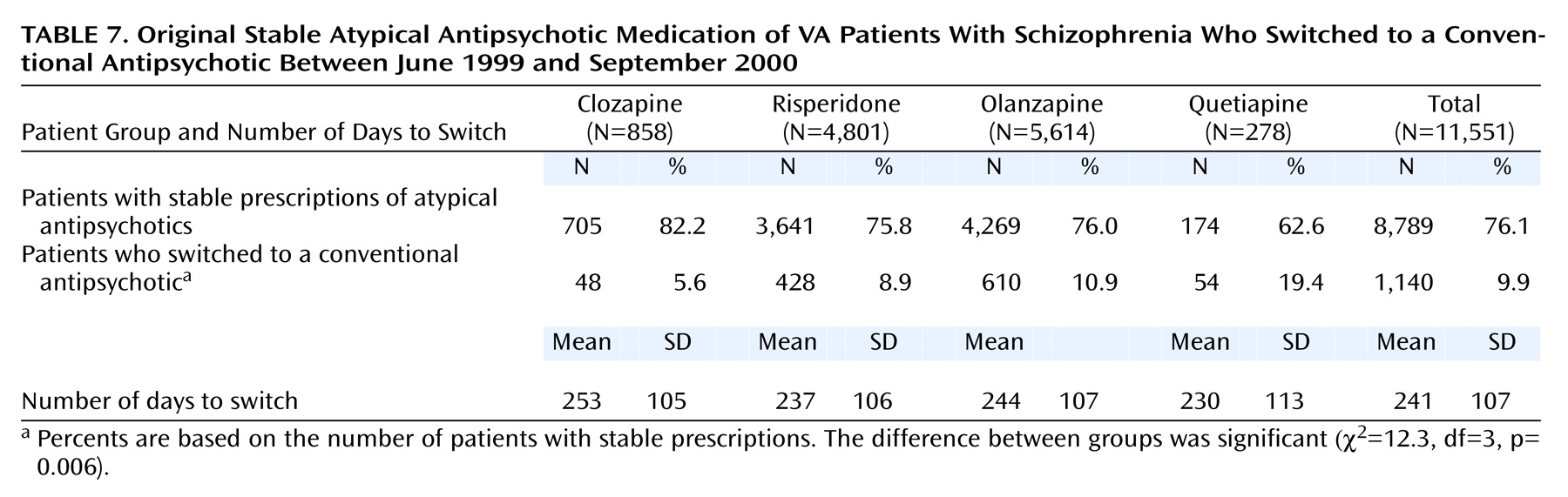
Table 7 shows additional, more specific information for patients who switched from an atypical to a conventional antipsychotic drug. A total of 1,140 patients switched from an atypical to a conventional medication. Patients whose original stable medication was quetiapine were the most likely to switch to a conventional antipsychotic, followed by those whose stable medication was olanzapine, risperidone, and clozapine. Although the differences between groups receiving different atypical antipsychotics in the proportion of patients who switched to a conventional antipsychotic are significant, the difference in the length of time on the original atypical medication before switching to a conventional was not (F=0.8, df=3, 1136, p=0.49).
Discussion
This study tracked the use of antipsychotic medications among patients with schizophrenia in a national health care system to determine the degree to which patients switch drug regimens and the predominate paths of switching. We found that of all patients in the original sample, those who were most likely to receive the same medication for 3 months were prescribed conventional antipsychotics, followed in order of stability by risperidone, olanzapine, clozapine, and quetiapine. Further, of those patients who had a stable antipsychotic regimen, almost one-quarter switched medications over the following year. Of patients who switched after a period of stability, most switched to olanzapine (35%), risperidone (29%), or a conventional medication (21%).
Of patients with a stable antipsychotic regimen who switched drugs, only 31% remained stable on the new medication. This suggests that if clinicians switched from one medication to another because they were not satisfied with how the patient was responding to the original drug, switching medications often did not help.
Other studies have tracked the increased use of atypical antipsychotics in the treatment of schizophrenia
(4–
6,
10–12). We expected to find that patients tended to switch away from conventional antipsychotic medications toward atypical antipsychotics and that once they started to take an atypical medication they would switch to another atypical medication. This would be consistent with a replacement process in which a clearly superior set of agents gradually replaced an inferior one.
However, we found that patients receiving a conventional medication were
more likely to maintain that prescription for 3 months and were about as likely or less likely to switch medications than those receiving three out of the four atypical drugs. Clozapine was the exception, probably because it is the only drug that has been shown to be more efficacious than conventional drugs among the more severely ill
(13). In addition, although 26% of the patients who had a stable regimen of conventional medication switched to an atypical drug, only a slightly smaller proportion of patients who had a stable regimen of an atypical medication (21%) switched to a conventional neuroleptic. This suggests that the growth in the use of atypical medications does not result from a simple replacement of the older drugs with the newer agents but from a more dynamic process of iterative decision making.
In most cases, patients with a stable antipsychotic therapy who switched to another medication eventually switched back to their original medication. This was more common for patients who switched to a conventional medication (70%) than for patients who switched to an atypical medication (45%) (χ2=8.82, df=1, p=0.003). In addition, of the patients who switched back to their original stable therapy, more than two-thirds did so within 30 days. This suggests that the pharmacotherapy of these patients was being augmented or that another agent was being tested rather than that their regimens were being changed altogether.
We were especially impressed by the consistency of results with respect to quetiapine. Very few patients received the drug (only 2% of the patients with schizophrenia who received an antipsychotic in June 1999). This is consistent with findings in other studies
(5,
6) and may be related to the fact that quetiapine was the newest of the medications to reach the market and clinicians may not have been as familiar with it as other drugs. Alternatively, it may be attributable to the suggestion that quetiapine’s manufacturer (Astra Zeneca) is newer to mental health markets than other manufacturers
(14).
Even among patients who received the drug, far fewer patients receiving quetiapine continued to take that drug for 3 months (45%) than patients who received other antipsychotic medications (range=73%–83%). In addition, patients receiving a stable regimen of quetiapine were also more likely to switch to another drug (37%) than patients with other stable medications (range=18%–26%). It is impressive that, over a 15-month period of pharmacotherapy, it was the medication for which patients were consistently most likely taken off and least likely to be prescribed. Studies of the effectiveness and risks associated with atypical antipsychotics have not shown conclusively that one is more effective or has fewer side effects than others. Quetiapine has been linked with weight gain and new-onset diabetes, but so have other atypical antipsychotic medications
(15–
18).
An alternative explanation might be that patients received relatively lower doses of quetiapine than other antipsychotics. In fact, an analysis of the average daily doses of these medications shows differences between agents relative to their Schizophrenia Patient Outcomes Research Team (PORT) recommended dosing ranges
(3). The median dose across all prescriptions in fiscal year 2000 was 200 mg/day for quetiapine (or about 8% of the way through the PORT recommended dosing range), compared with 400 mg/day for clozapine (56% of the PORT recommended range), 10 mg/day for olanzapine (33% of the PORT recommended range), and 4 mg/day for risperidone (50% of the PORT recommended range). The PORT recommended dosing range for quetiapine is wide compared with the other atypical medications (150–750 mg/day for quetiapine, compared with 150–600 mg/day for clozapine, 5–20 mg/day for olanzapine, and 2–6 mg/day for risperidone). Since quetiapine is relatively new to the market, physicians may have less experience in prescribing doses of quetiapine than they have for olanzapine or risperidone. Although there is evidence that quetiapine is as effective as haloperidol and that risperidone and olanzapine are slightly more effective than haloperidol
(19), one cannot conclude from this that quetiapine is less effective than risperidone or olanzapine. Our results provide a unique window on the processes by which clinicians “rate” more or less effective medications.
One might think that the proportion of patients who were switched to a newer medication might depend on the length of time that the medication has been available. The diffusion of an innovation over time has been described as an S-shaped curve: the proportion who adopt the new technology is small early on, the rate of adoption accelerates as it is more widely accepted, and then it slows again as all but the most firmly entrenched in the older technology have adopted the innovation
(20). We found that of the patients whose medication was switched, most were switched to olanzapine, which received FDA approval in 1996. Fewer patients were switched to risperidone, which was approved in 1994, and far fewer were switched to quetiapine, which was the most recently approved drug (1997). Such a pattern is consistent with an S-shaped curve: few patients may be switching to the newest of the drugs (quetiapine) because it is early in the diffusion process or to the oldest of the drugs (risperidone) because it is late in the diffusion process, while more patients may be switching to olanzapine because it is in the steep part of the diffusion curve. This pattern is not inconsistent with an S-shaped curve, but we cannot test this theory of diffusion without repeating our analyses at different points in time. If this theory is valid, it suggests that the time period over which the diffusion of new medications takes place is quite long.
An implication of our results is that there is a benefit to having all of these antipsychotic medications available to treat patients with schizophrenia. Formularies are inclined to limit the number of agents within a therapeutic class that are available to treat patients in the health care system. Although most patients whose medications were switched did not maintain a stable regimen on their new drug therapy, our results suggest that the new medication was beneficial for more than 30% of patients who were switched. In addition, in determining the optimal pharmacotherapy, clinicians and patients like to switch, and switch again. Therefore, restricting the availability of these medications may have a negative impact on patient well being. All of these medications are available on the VA formulary.
There are several limitations of this analysis that deserve comment. Some limitations relate to the fact that we used administrative records to examine prescribing patterns. Because these administrative data have very limited clinical measures, we cannot identify reasons behind decisions to switch medications or augment current pharmacotherapy. Patients may switch because side effects become intolerable, they are not responding to the medication, or for other nonclinical reasons. Patients may augment their current therapy with another drug to get them through a clinical exacerbation in which their symptoms become more severe. Without more detailed clinical data, we cannot determine the reasons for changing the pharmacotherapeutic regimen.
We also do not have data on the severity of illness. It is possible that patients who switch to the newer medications are more severely ill and have failed to respond to other medications. This might explain some of the differences we found with respect to quetiapine.
In addition, prescription drug records were not available before June 1999, which prevents us from repeating the analysis at different points in the diffusion of these medications. This limitation is also important because it is likely that the medication that a patient switches to depends on what medications have been tried previously.
Despite these limitations, it is clear that even among patients who receive a stable antipsychotic regimen, it is not uncommon for patients to switch or augment their antipsychotic pharmacotherapy, even changing from an atypical antipsychotic to a conventional drug. Although the use of atypical antipsychotics has grown considerably over recent years, our results suggest that the diffusion of these new medications is not a simple switch to the new medication and that finding the right medication for a particular patient is often a matter of iterative trial and error. Despite clinical evidence suggesting that atypical antipsychotics are at least as effective as conventional antipsychotics and have fewer side effects, individual patients can respond very differently to any particular medication. Therefore, atypical medications may not be the best pharmacotherapeutic option for every patient. There are many factors that influence the choice of schizophrenia pharmacotherapy that we still do not understand. More research is needed to understand how clinicians make decisions in actual practice. The method developed here may prove to be a useful application for aggregating clinical experience in large healthcare systems.