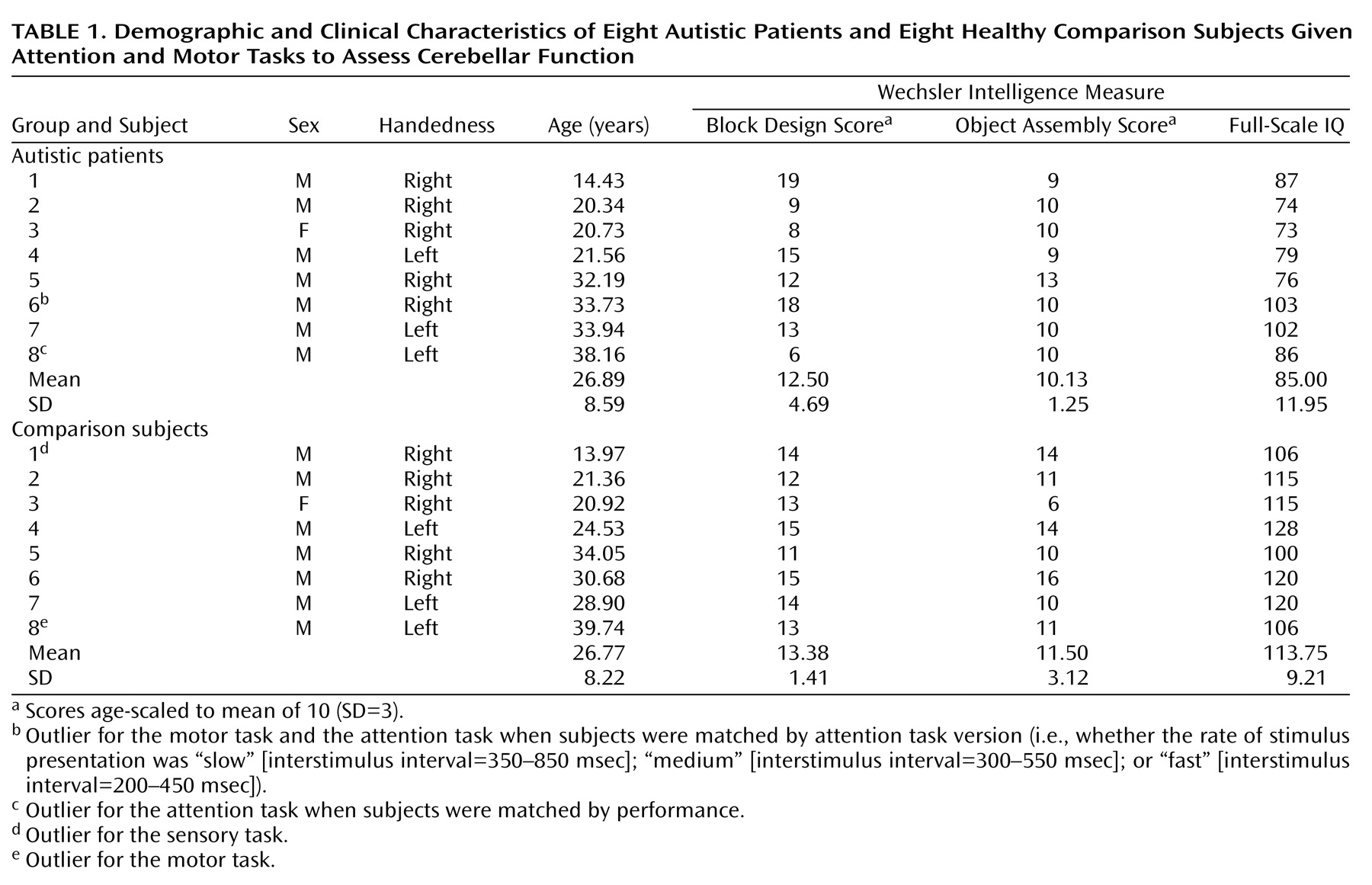
In recent years, the traditional view of cerebellar function has been seriously challenged. Long considered to be involved exclusively in motor coordination, new findings suggest that the cerebellum plays a role in multiple functional domains (e.g., references
1–
9). Such findings have emerged largely from functional neuroimaging studies. One such study used functional magnetic resonance imaging (fMRI) to demonstrate a dissociation between cerebellar regions involved in attention and those involved in a simple motor task
(5). Motor activation was localized to the anterior cerebellar hemisphere, whereas activation during a nonspatial selective attention task was localized to the superior posterior cerebellar hemispheres. Several other recent investigations have supported this finding by showing similar loci of cerebellar attention activation (e.g., references
7,
10–
13).
While these findings help to broaden our view of cerebellar function, they also imply that cerebellar pathology can effect a variety of
nonmotor functional deficits
(14). This is a crucial implication in light of mounting evidence for cerebellar involvement in various neurologic and psychiatric conditions, including autism
(15–
17), attention deficit hyperactivity disorder
(18), mood disorders
(19), obsessive-compulsive disorder
(20), and schizophrenia
(21,
22). Such evidence calls for a broader investigation of the functional consequences of cerebellar pathology. Autism provides a useful model for such an inquiry, for it is a condition in which cerebellar pathology is particularly well defined.
Ninety-five percent of autistic cerebella examined at autopsy have shown cerebellar anatomic abnormalities
(16,
23–
30), and in all but one case, the abnormality was a reduction in the normal number of Purkinje cells; molecular abnormalities in the autistic cerebellum have also been reported in all cases examined
(31–
33). The Purkinje cell is one of the principal cortical neurons in the cerebellum and the only source of output from the cerebellar cortex
(34). A crucial question, then, is how reduced numbers of this important element of circuitry ultimately impact cerebellar function. Studies that have used fMRI to show involvement of the normal cerebellum in attention would predict that cerebellar pathology is associated with attentional impairments, and such pathology-deficit associations have in fact been demonstrated in autism
(35,
36). However, what is not known is whether the autistic cerebellum is in fact functioning abnormally during attention tasks.
Results
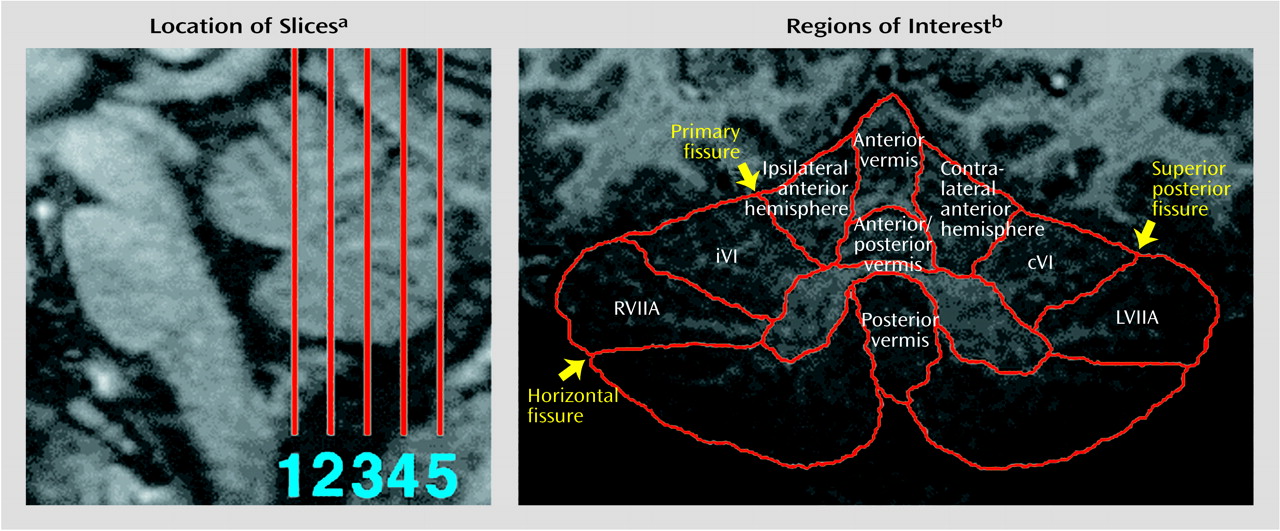
Cerebellar Volume and Region of Interest Anatomy
Mean cerebellar gray matter volume was 106.1 cm
3 (SD=22.4) for the autistic subjects and 109.9 cm
3 (SD=12.9) for the healthy comparison subjects. Thus, whole cerebellar volume was slightly (approximately 4%) smaller overall in the autistic subjects. This difference was not statistically significant (z=–0.42, p=0.34, one-tailed Wilcoxon rank sum test), but it was similar in direction and magnitude to the statistically significant difference in cerebellar volume recently found in much larger samples of autistic and normal subjects
(49). For seven out of nine regions of interest, anatomic volumes were also smaller in the autistic group. The degree of this difference ranged from 2% for left superior hemisphere lobule VIIa to 19% for the anterior hemisphere ipsilateral to the moving hand. This latter difference approached statistical significance (z=–1.54, p=0.06, one-tailed Wilcoxon rank sum test). In all other regions of interest, group differences were nonsignificant.
Behavioral Data
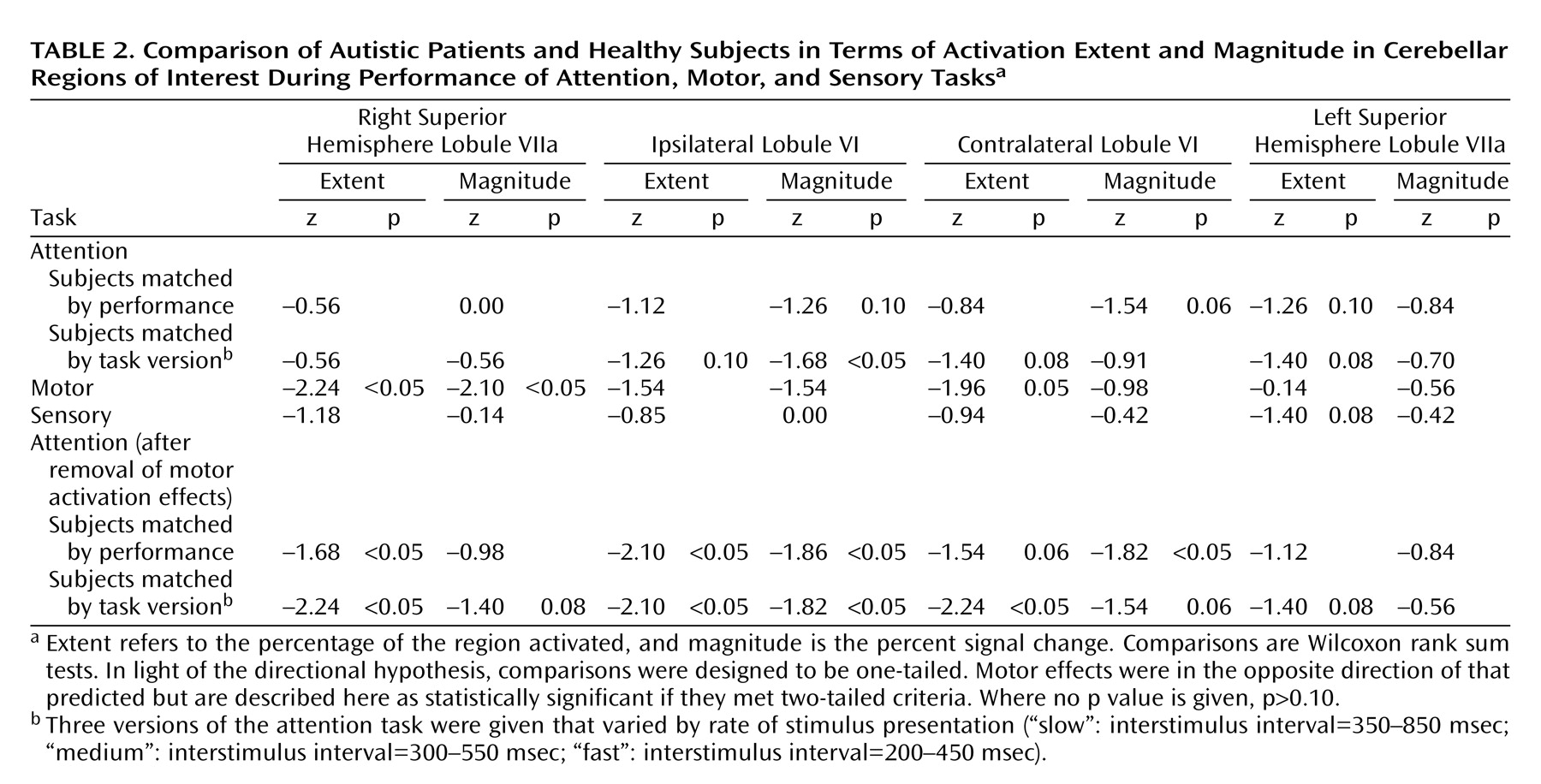
For the attention task, performance accuracy (i.e., percent correct target detections) did not differ between groups (autism mean=90.81, SD=6.88; healthy mean=91.98, SD=7.82) (z=–0.68, p=0.50, two-tailed Wilcoxon rank sum test). When subjects were matched by attention task version, autistic subjects were significantly less accurate than comparison subjects (mean=81.31 [SD=12.16] versus mean=91.98 [SD=7.82], respectively) (z=–2.03, p<0.05, two-tailed Wilcoxon rank sum test). As described earlier, the mean number of button presses across the four motor task blocks was recorded as an index of button press frequency, which did not differ significantly between groups (autism patients: mean=75.3, SD=18.8; healthy subjects: mean=58.9, SD=23.5) (z=–1.68, p=0.09, two-tailed Wilcoxon rank sum test). No subject pressed the button during the rest condition, and no other extraneous movements were observed.
Cerebellar Activation
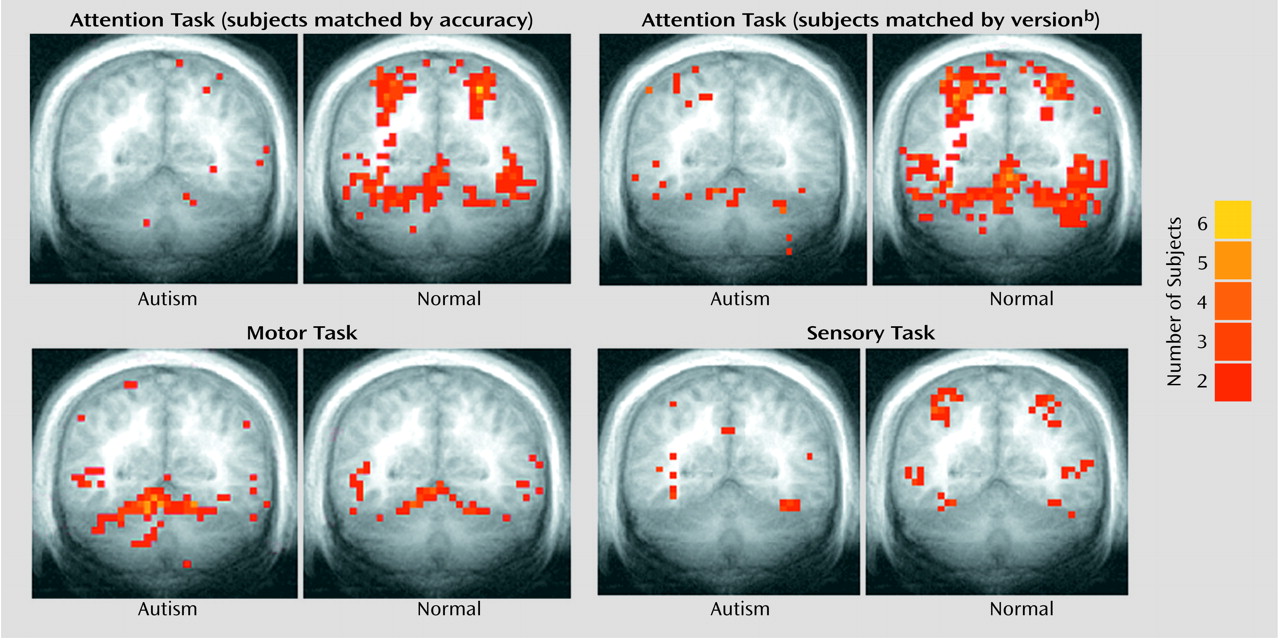
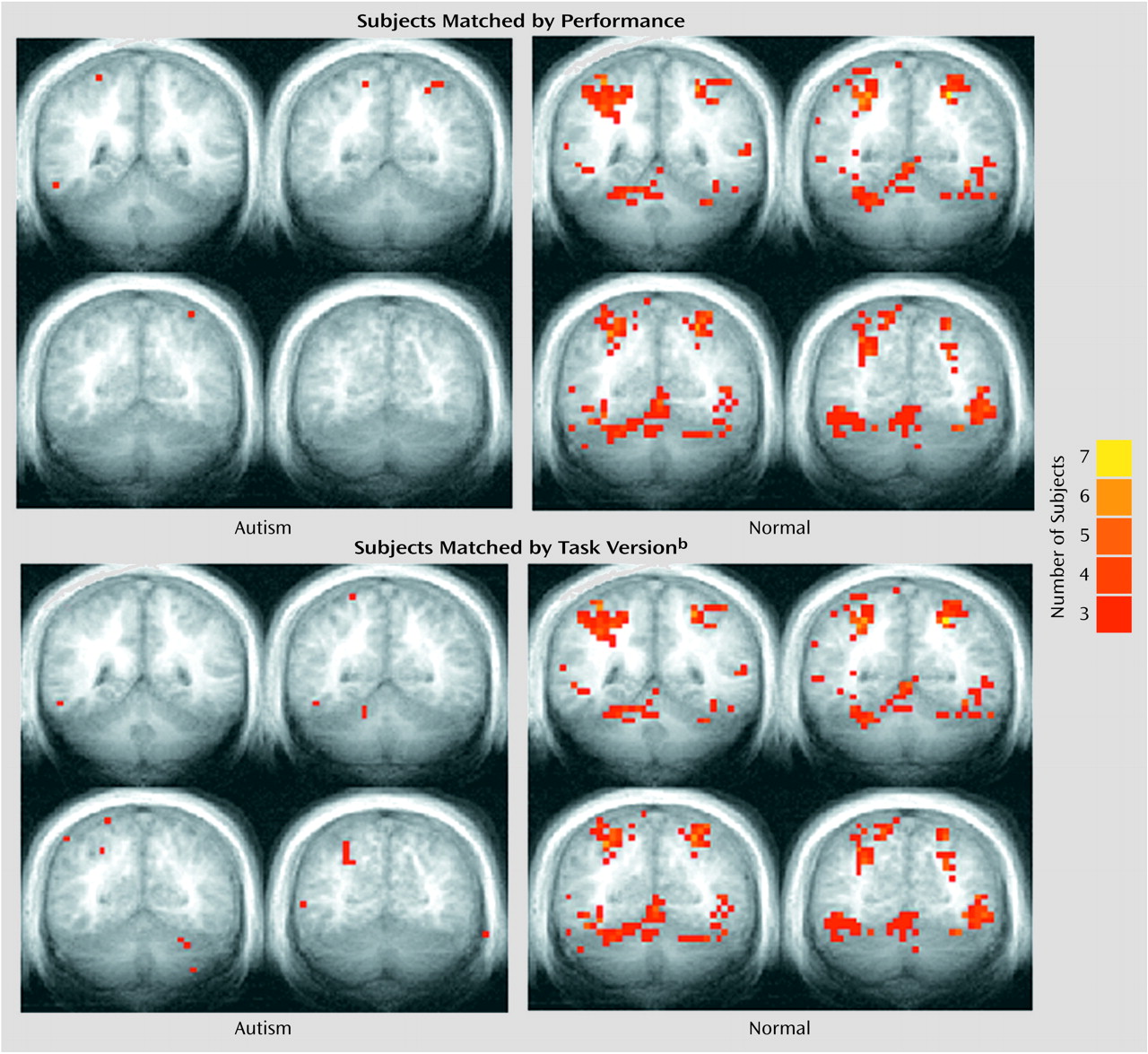
Figure 2 provides a summary of the activation results from the three tasks at a single slice location. As in our previous investigation
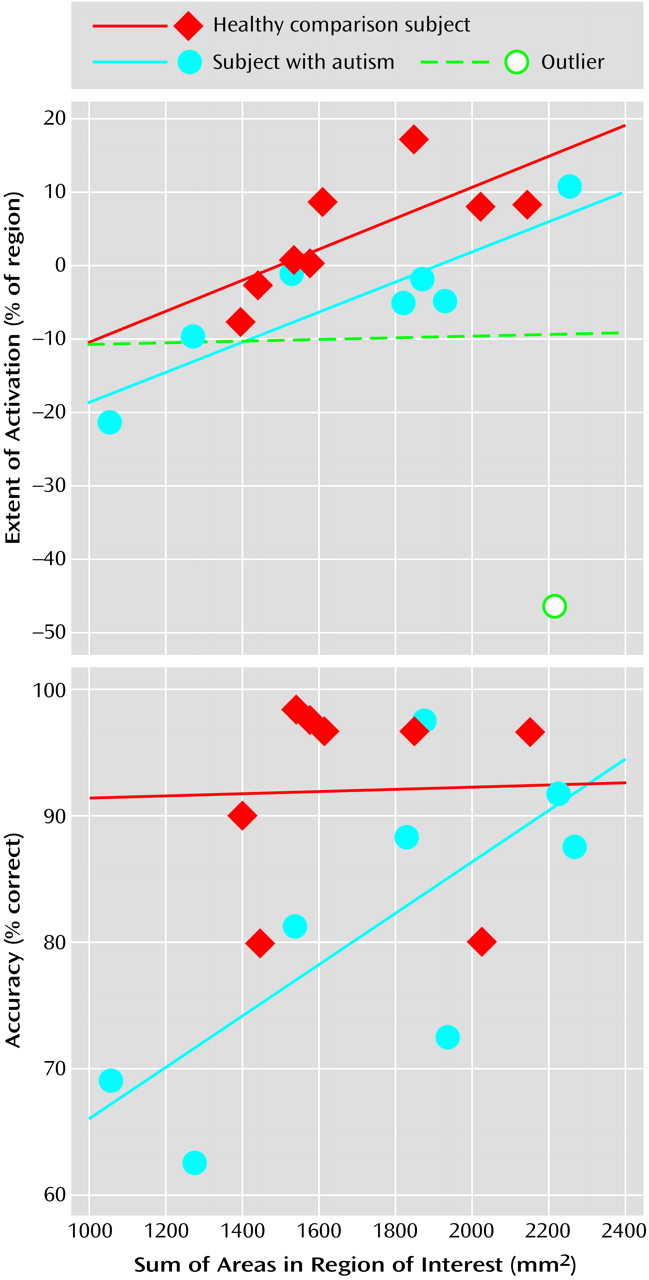
(5), healthy comparison subjects showed bilateral superior posterior cerebellar hemisphere activation during the attention task. A strong positive correlation between the size of cerebellar hemisphere lobule VIIa and activation extent in that same region was also observed in healthy and autistic subjects (
Figure 3). Moreover, even further support for the role of this region in attention came from the strong positive correlation seen in autistic subjects between the
anatomic size of this region and accuracy during the “fast” version of the attention task.
To address the question of normal differences in cerebellar cognitive and sensory activation, measures of attention and visual sensory activation were compared in the healthy subjects. Activation extent was significantly greater during the attention task in ipsilateral lobule VI (z=–2.1, p<0.05), contralateral lobule VI (z=–1.82, p<0.05), right superior hemisphere lobule VIIa (z=–1.69, p<0.05), and left superior hemisphere lobule VIIa (z=–1.82, p<0.05) (one-tailed Wilcoxon rank sum tests), while activation magnitude was significantly greater in ipsilateral lobule VI (z=–2.1, p<0.05) and left superior hemisphere lobule VIIa (z=–1.68, p<0.05) (one-tailed Wilcoxon rank sum tests).
Unlike their healthy counterparts, autistic patients showed minimal activation during the attention task (
Figure 2), and this was reflected in the quantitative region of interest effects.
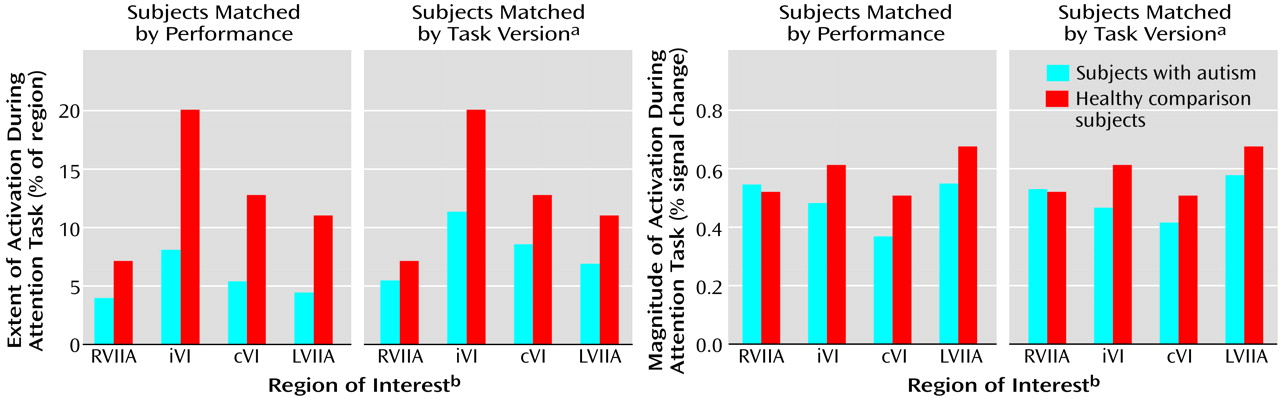
Figure 4 shows that whether subjects were matched for performance or task version, activation in almost all regions of interest was greater in the healthy comparison subjects.
Table 2 provides a summary of the group comparisons at all regions of interest for all tasks. For the performance match, the group differences in activation extent were not statistically significant, although this difference approached significance in the left superior hemisphere lobule VIIa. In terms of activation magnitude, the group difference approached significance in ipsilateral lobule VI and contralateral lobule VI. For the task match, activation extent was again not significantly reduced, although the difference approached significance in ipsilateral lobule VI, contralateral lobule VI, and the left superior hemisphere lobule VIIa. For activation magnitude, the group difference was statistically significant in lobule VI ipsilateral to the moving hand.
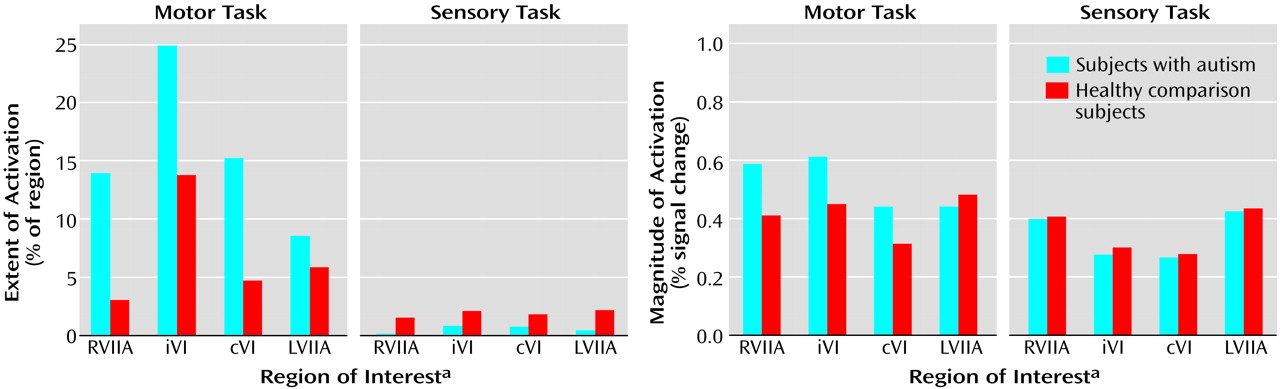
Group effects for the motor task were in the opposite direction from what was predicted and what was seen during the attention task. That is, autistic subjects showed a pattern of activation that was
more diffuse than that seen in the healthy subjects (
Figure 2 and
Figure 5). This group difference in activation extent met criteria for statistical significance in the right superior hemisphere lobule VIIa and approached significance in contralateral lobule VI. Motor activation magnitude in the cerebella of the autistic patients was also significantly greater than that seen in healthy subjects in the right superior hemisphere lobule VIIa (
Table 2). During the sensory task, trace amounts of cerebellar activation extent and magnitude were seen in both groups (
Figure 2 and
Figure 5).
Overall, the functional maps (
Figure 2) suggested that cerebellar attention activation was markedly reduced in autistic patients, while region of interest analyses depicted less dramatic differences that did not always meet criteria for statistical significance. This may have been due in part to limitations in statistical power and the presence of outliers. To explore this possibility, data were also analyzed with more powerful parametric tests (t tests) following the removal of outliers. Outliers (
Table 1) were identified by using the extreme Studentized deviate method
(51), which adjusts the critical z score for defining outliers according to sample size; for the present data, this was a z of 2.13. When analyzed in this manner, performance-matched activation extent and magnitude during the attention task were significantly reduced in autistic subjects in contralateral lobule VI, with the differences approaching significance in ipsilateral lobule VI and left superior hemisphere lobule VIIa. When matched by task version, activation was significantly reduced in left superior hemisphere lobule VIIa, with contralateral lobule VI and right superior hemisphere lobule VIIa approaching significance.
Group differences in attention activation may have also appeared less robust than expected because relative decreases in attention activation in the autistic subjects were obscured by simultaneous increases in motor activation. To address this possibility, each subject’s motor activation value was subtracted from his or her attention activation value in each region of interest.
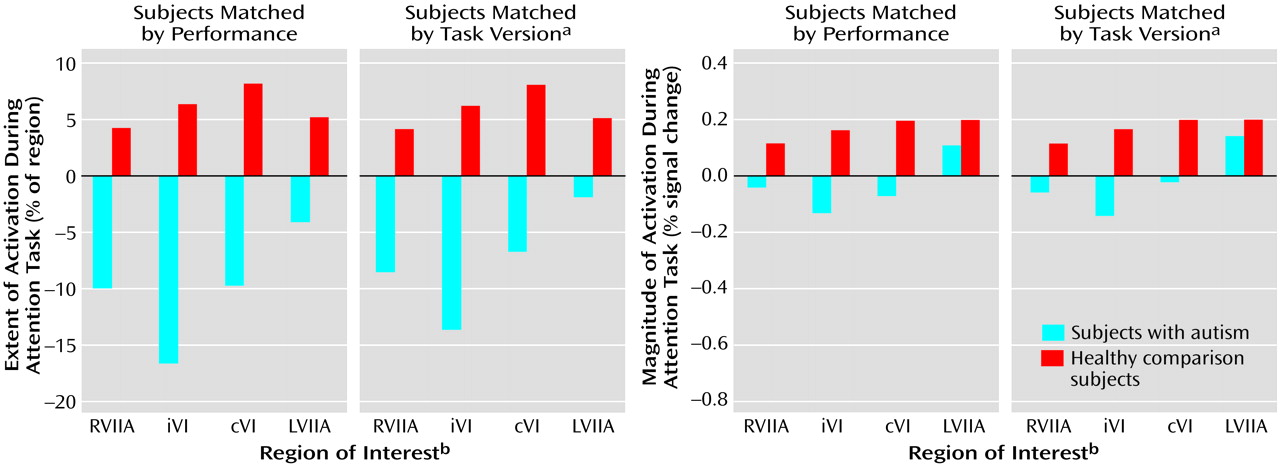
Figure 6 and
Table 21 show the results of the attention-minus-motor subtractions in the four regions of interest. When subjects were matched by performance, activation extent was significantly lower in the autistic subjects than in the healthy subjects in ipsilateral lobule VI and right superior hemisphere lobule VIIa, with the difference in contralateral lobule VI approaching significance. Activation magnitude was significantly lower in ipsilateral lobule VI and contralateral lobule VI. When subjects were matched by task version, activation extent in the autistic cerebellum was significantly reduced in ipsilateral lobule VI, contralateral lobule VI, and right superior hemisphere lobule VIIa, and this difference approached significance in left superior hemisphere lobule VIIa. Activation magnitude was significantly lower in ipsilateral lobule VI, with the difference in contralateral lobule VI and right superior hemisphere lobule VIIa approaching significance.
Figure 7 shows the effects of subtracting motor from attention activation on the functional maps. For both the performance and task matches, healthy subjects continued to show activation in the anterior cerebellum and bilaterally in superior posterior cerebellar hemispheres, whereas autistic subjects showed virtually no cerebellar activation.
Discussion
Although the cerebellum’s fundamental function remains a mystery, the functional domains that the cerebellum serves are remarkably diverse (for review, see reference
52). We previously demonstrated that the cerebellum is active during attention operations independent of motor involvement
(5). The present results replicate this finding, again demonstrating superior posterior cerebellar hemisphere involvement in nonspatial visual selective attention. The new finding that the size of cerebellar hemisphere lobule VIIa is strongly correlated with the amount of attention activation in that same region provides even further support for cerebellar involvement in attention operations. In contrast, minimal cerebellar activation was observed when subjects simply viewed visual stimuli but did not selectively attend or respond.
Attention-related cerebellar activation was lower in autistic patients than in healthy subjects whether or not groups were matched for performance, shedding new light on the cerebellar role in attention deficits in autism. Attentional impairments are among the most common forms of cognitive deficit in autism
(53). Thus, their basis is of great importance to clinicians, teachers, and parents of autistic individuals. Neurobehavioral studies conducted over the course of the past decade have shown that deficits in specific attention operations are associated with abnormalities of cerebellar anatomy. For instance, autistic individuals have difficulty rapidly reallocating attentional resources to new sensory modalities
(3,
54) and to new spatial locations
(9,
35,
36,
55,
56). Such findings are more prominent in autistic patients with greater abnormality of the cerebellum (i.e., hypoplasia), and they are also seen in patients with acquired neocerebellar lesions
(9,
54,
57). In addition, Lee and colleagues
(33) have recently described abnormal nicotinic receptor composition in the cerebella of autistic individuals, which, as pointed out by those authors, is of great interest given the role of the nicotinic receptor in attention
(58). Moreover, in the present study, decreased size of the right cerebellar hemisphere lobule VIIa in autistic subjects was associated with decreased accuracy in the attention task (
Figure 3). However, the question of whether the autistic cerebellum is functioning abnormally during attention tasks has remained unanswered, until now.
The present study showed that cerebellar functioning is, in fact, abnormally reduced during a selective attention task, even when groups were matched for performance. Such abnormal activation in the context of normal performance might suggest that the autistic brain has developed compensatory mechanisms for performing the attention task. However, an alternative interpretation is that a fundamental cerebellar function is not required to perform this task and thus is not “missed” by the dysfunctional autistic cerebellum. We have previously proposed that the fundamental function of the cerebellum is to learn predictive relationships among sequences of events so that whenever an analogous sequence begins to unfold in real time, the cerebellum can generate predictions about upcoming events and prepare whichever neural systems are expected to be needed to respond appropriately to such information
(3,
5,
14,
52,
59). Without this function, other systems continue to function but will do so suboptimally in situations where cerebellar prediction and preparation might otherwise aid performance.
In the attention task, the sequence of sensory events is randomly ordered and thus cannot be learned by the cerebellum. As such, the normal cerebellum might be active in attempting to learn and predict the sequence, but its activity cannot provide much useful preparatory output to neural systems involved in detecting and responding to upcoming target stimuli. Thus, although active, the normal cerebellum is not effectively aiding the rest of the central nervous system and therefore does not have a noticeable advantage over the autistic cerebellum in this context. In fact, autistic and neocerebellar lesion patients are typically not impaired on attention tasks similar to the one employed here
(3). Why, then, were autistic subjects impaired on certain versions of the attention task in this study? In the fastest version of the attention task, interstimulus intervals ranged from 200 to 450 msec, but in previous studies that used a similar task, interstimulus intervals ranged from 450 to 1450 msec. Perhaps at this new high rate stimuli outpaced the basic sensory tracking abilities of the dysfunctional autistic cerebellum. That is, although the loss of preparatory output may not impede performance in this context, deficient sensory tracking
(60) might do so when stimuli appear at such a rapid rate. Of course, it is also possible that supratentorial dysfunction played a role in this impaired performance, and as can be seen in the functional maps (
Figure 2 and
Figure 7), activation in cerebral cortex was also reduced in the autistic subjects.
The simplest and most straightforward interpretation regarding changes in cerebellar activation in autism is that a decreased volume of viable tissue due to a reduction in Purkinje cells will lead to abnormally reduced functional activation. While this might seem sufficient to explain the attention findings, it is clearly not sufficient when one considers the unexpected activation
increases seen during a simple motor task. Although the autistic individuals pressed the button at a slightly faster rate than their healthy counterparts, this did not account for the large difference in activation, and button press frequency was in fact not correlated with greater activation in these subjects. One possible alternative explanation for the divergent findings is derived from the “crowding” concept of neuroplasticity
(61). This concept was born out of the fact that patients with early lesions of the left cerebral hemisphere can show preserved language at the cost of other functions typically subserved by the right hemisphere. The notion is that intact tissue in the right hemisphere “takes over” the language functions but other functions are “crowded out” in the process.
One view regarding crowding is that more primitive functions, both phylogenetically and ontogenetically, are more likely to be spared and thus more likely to crowd out later developing ones
(62). In the cerebellum, basic motor functions tend to be subserved by phylogenetically older regions (e.g., the paleocerebellum), while more advanced cognitive operations tend to involve newer regions (i.e., the neocerebellum) that evolved in parallel with parts of the cerebral cortex involved in those same functions
(63). These more recently evolved regions of the cerebellum include superior posterior hemisphere regions that are active during attention tasks. We suggest that an early loss of Purkinje neurons might cause more primitive functions normally subserved by paleocerebellar regions to be displaced into the neocerebellum at the cost of tissue that subserves cognitive functions such as attention and language. Functional activation during simple motor tasks is normally most prominent in the paleocerebellum ipsilateral to movement
(5,
64,
65). However, in the autistic cerebellum, such activation spreads to include contralateral and posterior regions not normally associated with simple motor tasks, including neocerebellar areas normally involved in attention. This might reflect such motor functions being taken over by newer regions of the cerebellum due to loss of Purkinje cells in the older ones. Reduced attention activation in these regions thus may reflect dual circumstances of Purkinje cell reduction (i.e., impaired function due to abnormal circuitry and crowding out of what remains by more primitive functions). In this context, it is interesting to recall that the region of interest with the greatest reduction in anatomical size was in fact the anterior hemisphere ipsilateral to the moving hand, the paleocerebellar region typically involved in simple motor tasks.
The present study is but a first look at the relationship between developmental anatomic defect and patterns of fMRI cognitive activation in the human cerebellum. Much more needs to be done to better understand this relationship. Cerebellar anatomic abnormality, whether developmental or acquired, often does not occur in isolation. Therefore, the interaction between multiple loci of abnormality, and how this relates to functional impairment, is an important area of investigation. In the present study, functional maps indicated that autistic subjects have additional reductions in activation of cerebral cortex, particularly in the parietal lobes (manuscript in progress). While we cannot rule out the possible influence of such changes on cerebellar activation through rich cerebro-ponto-cerebellar projections
(66), one must also consider the potential influence of cerebellar functional changes on reductions in cerebral activation via reciprocal cerebello-thalamo-cortical projections. It is to be hoped that these and other questions will be addressed by future studies of autism and other disorders, ultimately leading to a broader understanding of the functional implications of cerebellar anatomic defect.