Memory is a highly complex process that involves several brain structures as well as the role of several neurotransmitters. Considerable efforts have been devoted to the development of a successful neurotransmitter-based pharmacotherapy for the memory dysfunction found in neurological and psychiatric diseases. Cholinergic and glutamatergic systems have been linked to cognitive processes such as attention, learning, and mnemonic function
(1,
2). However, accumulating evidence from recent studies indicates that other neurotransmitter systems, such as the serotonergic (5-HT) system, play a role in behaviors that involve high cognitive demand
(3).
Among the 5-HT receptor subtypes shown to play a role in various learning and memory models, the 5-HT
1A receptors are of particular interest. This receptor is characterized by its high concentration in the limbic system
(4), such as the hippocampus, which is known to play an important role in learning and memory
(5,
6). It interacts with other neurotransmitter systems, such as in the glutamatergic and cholinergic systems
(7,
8). In animal studies, specific agonists and antagonists of the 5-HT
1A receptor showed a consistent role for this receptor in learning and memory function
(4).
Several lines of evidence have suggested that the 5-HT
1A receptor may be involved in the pathophysiology of schizophrenia and the mechanism of action of atypical antipsychotic drugs
(9–
11). Atypical antipsychotic drugs with partial agonistic property on 5-HT
1A receptors were reported to improve verbal memory function in patients with schizophrenia
(12–
14). The use of a small dose of the 5-HT
1A agonist tandospirone in patients with schizophrenia has been reported to improve verbal memory
(15,
16). However, the exact mechanisms underlying these effects are not yet well defined, and the relationship in vivo in humans between the 5-HT
1A receptors and cognitive function across levels from underlying brain systems to neurons and cellular events within these systems has not been clarified.
To gain an understanding of this relationship, we performed positron emission tomography (PET) scans using [
11C]WAY-100635
(17,
18) to examine the 5-HT
1A receptor, assessing the relationship between regional receptor binding and memory function. To interpret the pharmacological implications, we administered the 5-HT
1A agonist tandospirone
(19–
21) to subjects and investigated the effect of the stimulation of 5-HT
1A receptors on cognitive function together with the neuroendocrinological response of growth hormone (GH) and body temperature, which reflect postsynaptic 5-HT
1A receptor activity
(22–
24).
Results
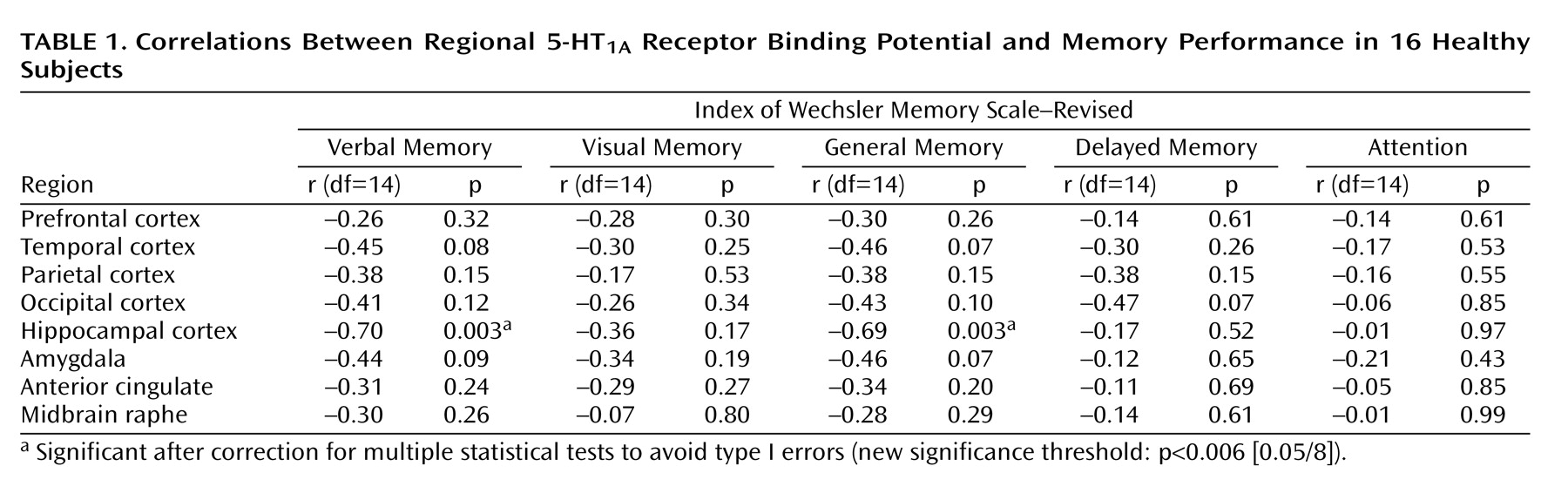
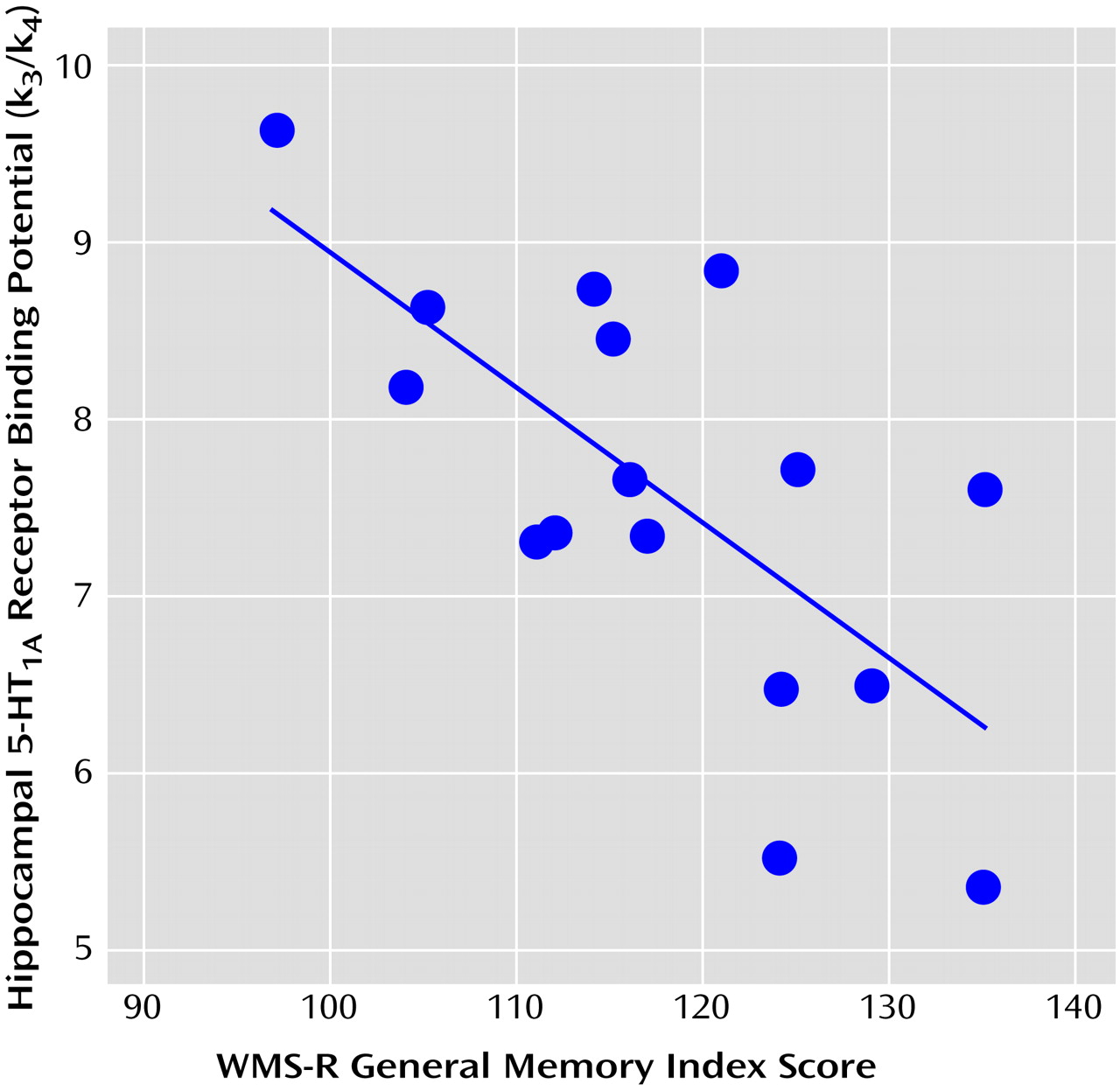
A significant negative correlation was observed between 5-HT
1A receptor binding potential in the hippocampus and the verbal and general memory indices of WMS-R
(25,
26) (
Table 1 and
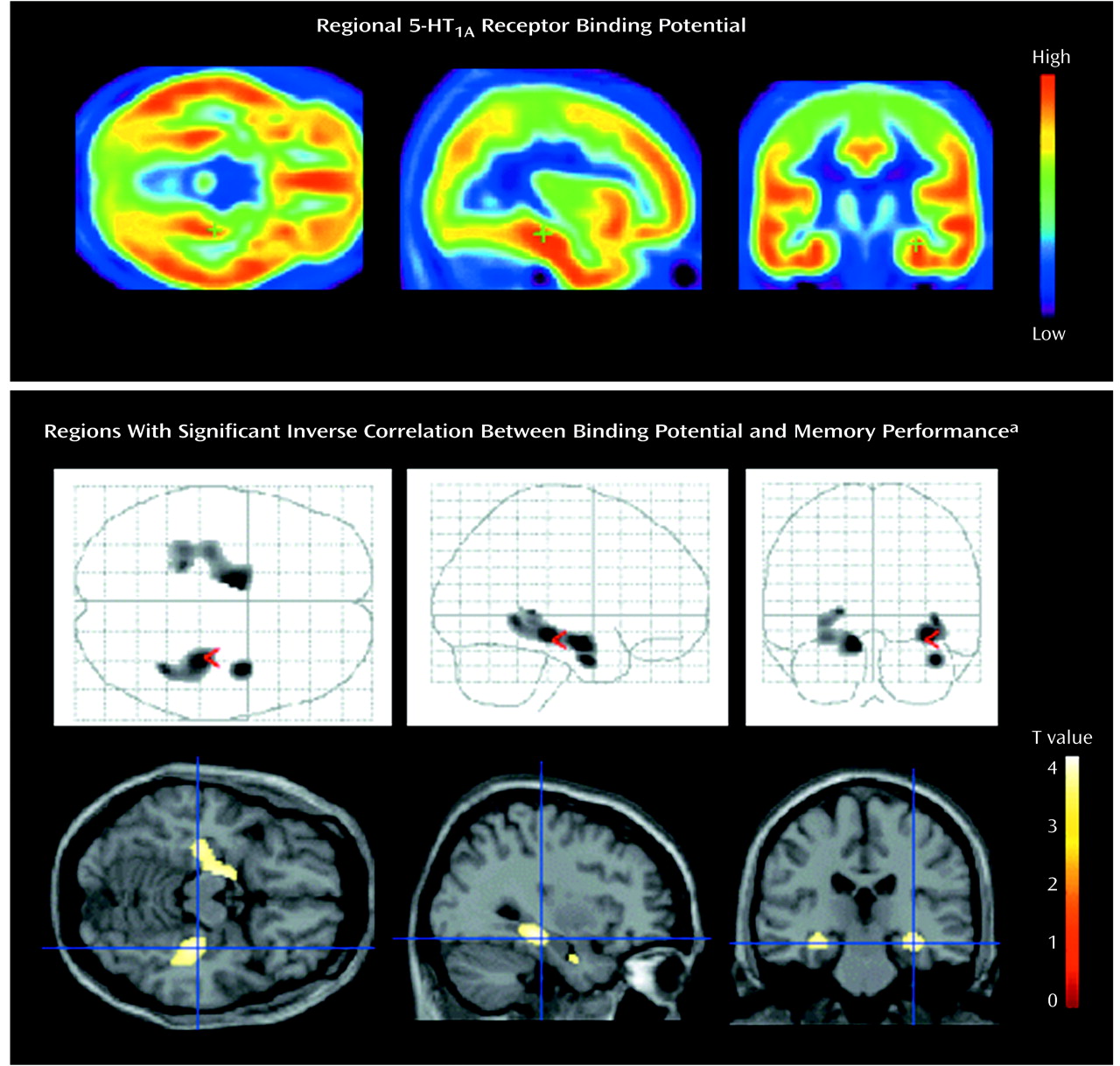
Figure 1). No significant correlations were observed among any other indices and regions, and there was no age effect on binding potential in any region. The significance of this correlation was consistent with the finding from the analysis using statistical parametric mapping
(31), which revealed significant correlation in the bilateral hippocampus (Montreal Neurological Institute coordinates: x=–30, y=–22, z=–14 in the left side; x=34, y=–28, z=–12 and x=40, y=–4, z=–28 in the right side) (
Figure 2).
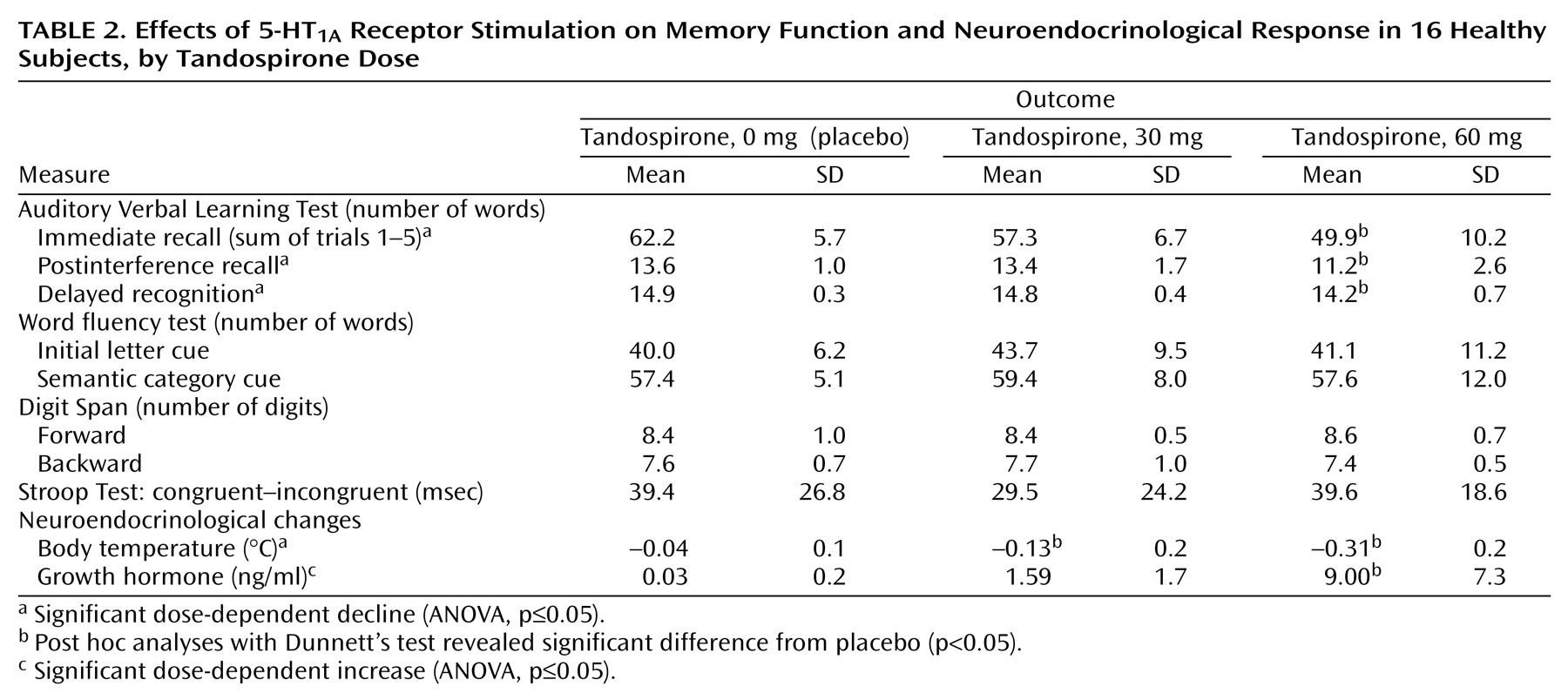
As seen in
Table 2, the effect of the stimulation of 5-HT
1A receptors with tandospirone on explicit memory function, as measured with the Auditory Verbal Learning Test
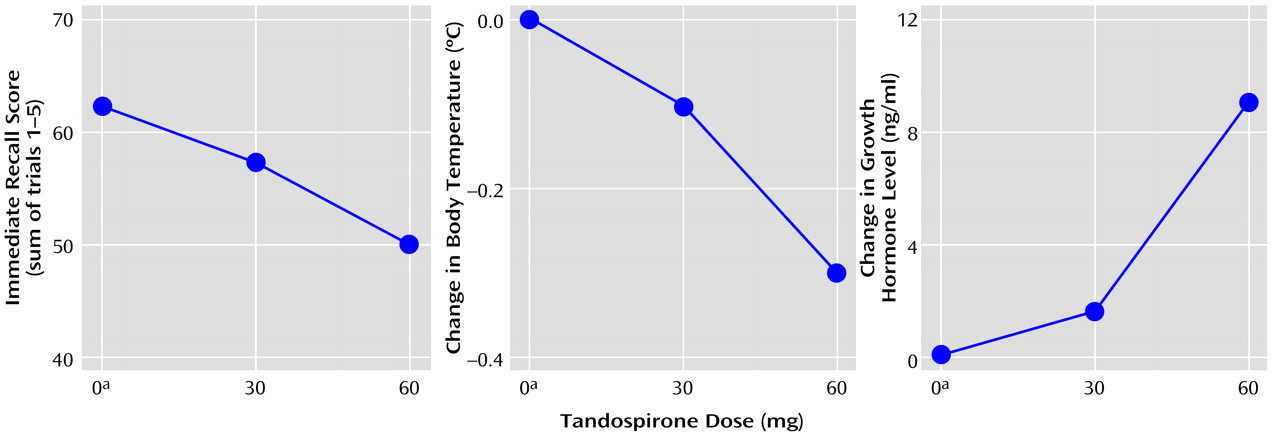
(33), revealed a significant dose-dependent decline in performance (immediate recall: F=7.33, df=2, 16, p=0.005; postinterference recall: F=4.47, df=2, 16, p<0.03; delayed recognition: F=3.65, df=2, 16, p=0.05). Post hoc analysis revealed that the placebo condition differed significantly from the 60-mg tandospirone condition in immediate recall (t=12.3, df=16, p=0.003), postinterference recall (t=2.22, df=16, p<0.04), and delayed recognition (t=0.67, df=16, p<0.05). On the other hand, there was no significant drug effect on the performance of the word fluency task (initial word fluency: F=2.31, df=2, 16, p=0.13; category word fluency: F=2.22, df=2, 16, p=0.14), digit span (forward: F=0.17, df=2, 16, p=0.85; backward: F=0.26, df=2, 16, p=0.77) or the Stroop task (F=1.37, df=2, 16, p=0.28).
Neuroendocrinological examinations revealed that tandospirone dose-dependently decreased body temperature (F=17.84, df=2, 16, p=0.008) and increased GH (F=12.04, df=1.1, 8.7, p=0.007). Post hoc analysis revealed that the placebo condition differed significantly from the 30- and 60-mg tandospirone conditions regarding change in body temperature (30 mg: t=0.16, df=16, p<0.04; 60 mg: t=0.33, df=16, p=0.0001) and from the 60-mg condition for GH (t=9.0, df=16, p=0.001). The changes in mean outcome parameters of memory function paralleled those of body temperature and GH (
Table 2 and
Figure 3), although there were no statistically significant correlations among these measures because of their large interindividual variabilities, as indicated by the standard deviations.
Discussion
The present results showed a significant negative correlation between explicit memory function and 5-HT
1A receptor binding localized in the bilateral hippocampal area where the postsynaptic 5-HT
1A receptors are enriched
(3). Although age-related hippocampal atrophy has been reported
(37), within the age range of the present study there was no age-related reduction of hippocampal [
11C]WAY-100635 binding (r=–0.10, df=14, p>0.70). Furthermore, the present results indicated that decreased [
11C]WAY-100635 binding correlated with better memory performance, which was opposite to the reported aging effect. Partial volume effect is unlikely to account for the results presented here.
Since [
11C]WAY-100635 binding was not sensitive to either acute or chronic changes of endogenous serotonin
(38), our finding might be attributable to the density of 5-HT
1A receptors. The results point to an association between high hippocampal 5-HT
1A receptor density in subjects and lower explicit memory ability. Furthermore, we found that the administration of tandospirone, a specific 5-HT
1A agonist, dose-dependently impaired explicit verbal memory, whereas other cognitive functions showed no significant changes. The change in memory function paralleled those of body temperature and GH, which were reported to be induced by the stimulation of postsynaptic 5-HT
1A receptors
(23,
24).
When these observations are taken together, it appears evident that the activity of postsynaptic 5-HT1A receptors in the hippocampus has an inhibitory influence on human explicit memory function. Although it was not clear whether individual variability in 5-HT1A receptor densities in the hippocampus is wholly genetically determined or whether it is subject to environmental influences such as stress factors, one could postulate that it is a cause of the individual differences in memory ability.
In animal models, it has been demonstrated that systemic and intrahippocampal injections of 5-HT
1A receptor agonists induced memory and learning impairment
(39,
40) and that the stimulation of 5-HT
1A receptors resulted in neuronal hyperpolarization and inhibition of neuronal activity in the hippocampus
(41). Postsynaptic 5-HT
1A receptors are predominant on pyramidal neurons
(4), and it is reasonable to consider that the negative influence of postsynaptic 5-HT
1A receptors on memory function may reflect the decrease in pyramidal cell activity by the inhibitory effect of 5-HT or 5-HT
1A agonist on pyramidal neurons in the hippocampus. It was also reported that 5-HT
1A antagonists ameliorated the learning and memory impairment induced by muscarinic and NMDA receptor antagonists in animals
(42,
43). Cellular localization of 5-HT
1A receptors has been demonstrated on both cholinergic and glutamatergic neurons
(7,
8), and the antagonism of 5-HT
1A receptors may increase their neuronal activity.
On the other hand, in an animal study with 5-HT
1A-knockout mice, worsening in learning and memory tests has been reported
(44). However, a complete deficit of 5-HT
1A receptors may also result in the loss of several neural networks that would be important in control learning and memory
(45), and some developmental abnormalities in the formation of neural networks certainly cannot be excluded in knockout mice
(46). Chronic administration of tandospirone was reported to improve verbal memory in schizophrenic patients treated with haloperidol
(15,
16). However, the lower dose of agonist used in their study might preferentially activate presynaptic 5-HT
1A receptors with higher sensitivity located on raphe neurons
(47), which provide a feedback regulation of the 5-HT system and decrease the release of 5-HT at postsynaptic sites
(48). Atypical antipsychotic drugs that exhibit partial agonism at 5-HT
1A receptors may preferentially activate presynaptic 5-HT
1A receptors with higher sensitivity while blocking their postsynaptic counterparts
(49). Their memory improvement properties appear to be mediated, in part, by their antagonistic effect on postsynaptic 5-HT
1A receptors in the hippocampus.
Our finding provides the first documentation of the human in vivo functional molecular mapping of explicit memory that is supported by a neuroendocrinological response. The results show that the postsynaptic 5-HT1A receptors localized in the hippocampal formation have a negative influence on explicit memory function. Our findings give rise to the possibility that the antagonistic effect of postsynaptic 5-HT1A receptors in the hippocampus leads to improvement of human memory function. Drugs that work as antagonists on postsynaptic 5-HT1A receptors may be favorable for improved control of memory impairment.