In recent years, there has been growing appreciation that the original monoamine hypotheses do not fully account for the neurobiology of depression or the mechanisms of action of effective treatments. Several lines of research implicate the glutamatergic system in the pathophysiology and treatment of depression, including the delayed, indirect effects of many antidepressants on the glutamatergic system
(1), as well as the antidepressant effects of
N-methyl-
d-aspartate receptor antagonists in animal models of depression
(2) and in humans
(3). It is also noteworthy that lamotrigine, which inhibits glutamate release, has antidepressant effects in humans
(4). Furthermore, there is increasing evidence that mood disorders are associated with regional reductions in CNS volume, possibly resulting from impairments of structural plasticity and cellular resilience (reviewed in reference
5). Notably, the glutamatergic system has been implicated in regulating neuronal plasticity and cellular resilience in a variety of neuropsychiatric disorders.
Glutamate-mediated impairments of plasticity have been implicated in neurodegenerative diseases, and several treatment strategies have been implemented to reduce excess CNS glutamate in these diseases
(6). One approach involves the use of riluzole, a glutamate-modulating agent
(7), which is approved by the Food and Drug Administration for the treatment of amyotrophic lateral sclerosis
(8).
The objective of this study was to determine the efficacy and safety of the glutamate-modulating agent riluzole in the treatment of recurrent major depression.
Method
Men and women ages 18 to 70 years, inpatients or outpatients, with a diagnosis of recurrent major depressive disorder without psychotic features as diagnosed by means on the Structured Clinical Interview for Axis I DSM-IV Disorders—Patient Version
(9) were eligible to participate. Subjects were required to have a Montgomery-Åsberg Depression Rating Scale
(10) score of ≥20 at screening and at start of medication treatment (baseline). All subjects were required to have previously been unresponsive to an adequate antidepressant trial. The staging of treatment resistance was as follows: stage 1=nonresponse to at least one adequate trial of one major class of antidepressants; stage 2=nonresponse to at least two adequate trials of at least two distinctly different classes of antidepressants; stage 3=stage 2 plus nonresponse to an adequate trial of a third distinct class or augmentation with lithium; stage 4=stage 3 plus nonresponse to an adequate trial of a fourth antidepressant from a distinct class or an additional augmentation strategy; and stage 5=stage 4 plus nonresponse to a course of bilateral ECT (staging was modified from reference
11). The adequacy of antidepressant trials was determined with the Antidepressant Treatment History Form
(12).
The patients were free of comorbid substance abuse or dependence (except for nicotine dependence) for 3 months, free of other axis I disorder diagnoses for the past 12 months (a lifetime axis I diagnosis was permitted), free of acute medical illness, and judged clinically not to be a serious suicidal risk. Subjects with a history of spontaneous or antidepressant-induced hypomania or mania were excluded. Written informed consent was obtained from all subjects after the procedures had been fully explained. A trained mental health professional that was not the treating clinician administered the ratings. High interrater reliability for the Montgomery-Åsberg Depression Rating Scale (ICC=0.88) and the Hamilton Anxiety Rating Scale (ICC=0.94) was obtained. After a 1-week drug-free period (2 weeks if taking a monoamine oxidase inhibitor), the participants were treated openly for 6 weeks. Subjects who had a greater than 20% decrease in score on the Montgomery-Åsberg Depression Rating Scale during the 1-week drug-free period were excluded. The riluzole dose was started at 50 mg b.i.d. and was increased after 2 weeks at the rate of 50 mg a week, as tolerated, up to a maximum of 200 mg/day. The dose range chosen for this study was based on previous trials of patients with amyotrophic lateral sclerosis and ranged from 50 to 200 mg/day. Zolpidem, 5–10 mg/day, was given as needed for insomnia but not within 8 hours of rating. The subjects were evaluated on a weekly basis with the Montgomery-Åsberg Depression Rating Scale, the Clinical Global Impression (CGI) severity scale, and the Hamilton anxiety scale. Clinical response was defined as a 50% decrease in score on the Montgomery-Åsberg Depression Rating Scale from baseline, and clinical remission was defined as achievement of a score of ≤10 on the Montgomery-Åsberg Depression Rating Scale. No individual or group therapy was permitted during the trial. Treatment compliance was monitored by capsule counts.
Results
Of 26 subjects screened, 19 patients (nine men and 10 women; mean age=43.1 years, SD=11.7) entered the study, the majority of whom were outpatients (73.7%). The patients received riluzole at a mean dose of 168.8 mg/day (SD=27.2) (84.2% took a dose of 150 mg/day or more) for a mean duration of 5.4 weeks (SD=3.7). The mean age at onset of the illness was 23.9 years (SD=12.6), the mean number of lifetime episodes of depression was 8.5 (SD=11.3), and the mean duration of the current episode of depression was 5.4 months (SD=3.7). Most of the patients were of stage 2 or greater treatment resistance (stages 1=47%, 2=31%, 3=11%, 4=0%, and 5=11%). Two subjects had previously failed to respond to lamotrigine therapy.
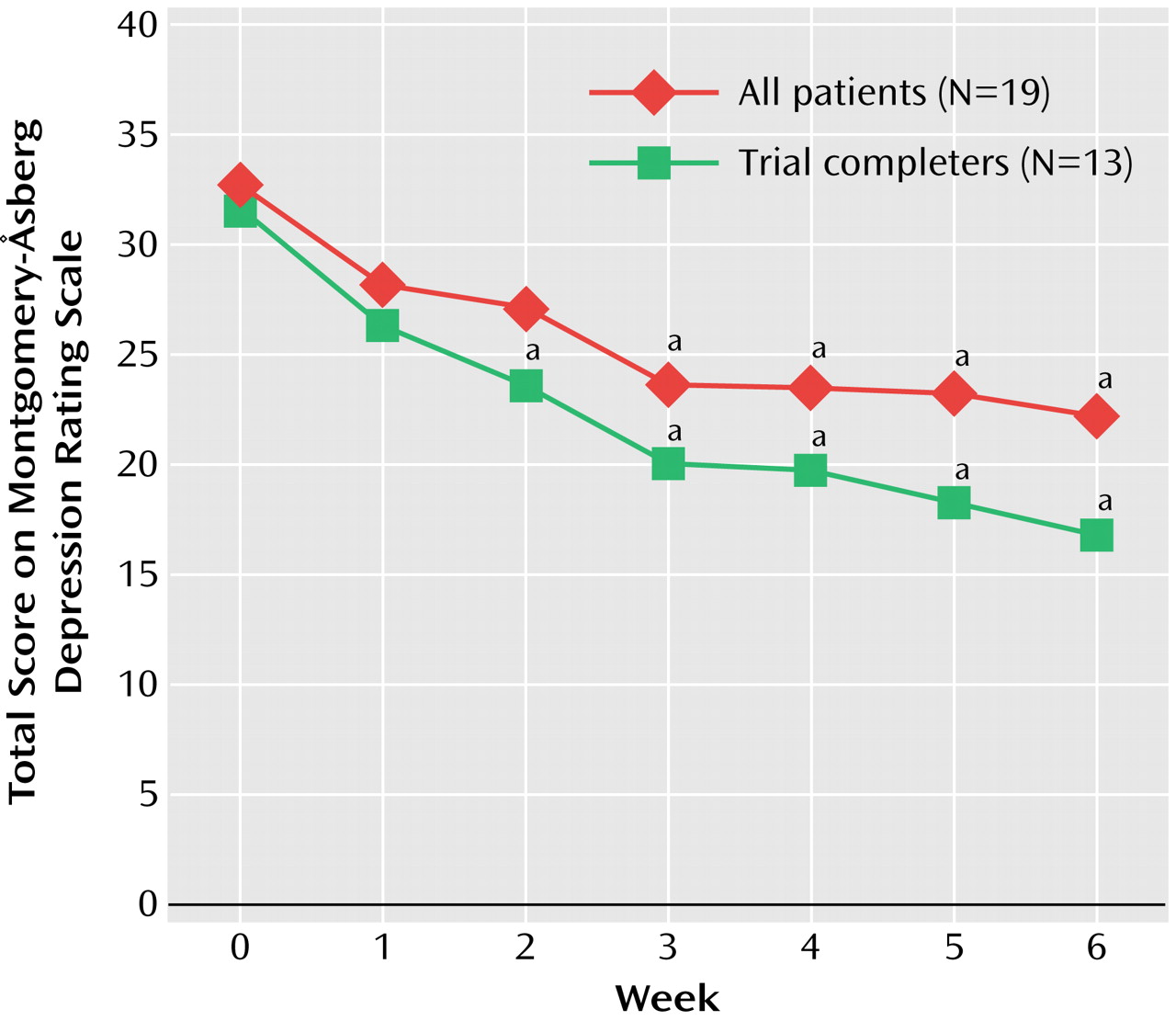
Sixty-eight percent (N=13) of the subjects completed the 6-week trial. The reasons for discontinuation were adverse events (N=3) (one with increased values on a test of liver function, one with malaise, and one with nausea and vomiting), nonresponse (N=2), and withdrawn consent (N=1). Data were analyzed on an intent-to-treat basis. Dunnett’s multiple range test was used to compare weekly ratings to those at baseline. Significant improvement in score on the Montgomery-Åsberg Depression Rating Scale occurred on weeks 3 through 6 for all patients (F=5.44, df=2, 39, p=0.007) and weeks 2 through 6 for trial completers (F=6.70, df=2, 25, p=0.004) (
Figure 1). CGI severity scale and Hamilton anxiety scale scores also improved significantly in weeks 3 through 6 for all patients (F=6.75, df=1.9, 34, p=0.04) and trial completers (F=7.62, df=2.0, 24, p=0.003, and F=5.77, df=1.2, 26, p=0.0007) on the CGI severity scale and Hamilton anxiety scale, respectively. The Hamilton anxiety scale score decreased from a baseline mean of 19.3 (SD=7.7) to a mean of 13.8 (SD=9.4). Response rates (50% decrease in score on the Montgomery-Åsberg Depression Rating Scale) at week 6 for all patients and trial completers were 32% and 46%, respectively. Remission rates (score on the Montgomery-Åsberg Depression Rating Scale <10) at week 6 for all patients and completers were 21% and 31%, respectively. Age, gender, clinical features, and dose of riluzole had no effect on change in scores on the Montgomery-Åsberg Depression Rating Scale. Response rates were higher in subjects receiving a dose of 150 mg/day (response rates at 100 mg/day were one-third [33%]; at 150 mg/day, one-half [50%]; and at 200 mg/day, one-eighth [13%]), although these differences were not statistically different. There were no differences in demographic or clinical variables between responders and nonresponders.
The most common adverse events during the trial were headache (58%), gastrointestinal distress (nausea or vomiting) (43%), decreased salivation (47%), constipation (32%), and tension or inner unrest (26%); similar side effects have been observed with riluzole in trials of patients with amyotrophic lateral sclerosis
(8). No serious adverse events were noted. One subject was dropped from the study because of an increase in values in liver function tests to three times the upper normal limit. This subject was asymptomatic for hepatic dysfunction, and liver function values returned to normal shortly after discontinuation of riluzole. There was no relationship between the dose of riluzole and adverse events or changes in laboratory test values.
Discussion
In this open-label study, riluzole was associated with antidepressant effects in individuals with treatment-resistant major depression. The response and remission rates in the present study are comparable to those of other antidepressants in studies of treatment-resistant depression
(13,
14).
Riluzole has been postulated to exert its effects on the glutamatergic system by means of two distinct mechanisms: through the inhibition of voltage-dependent sodium channels, resulting in the reduction of glutamate release, and also by means of indirect effects on α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid/kainate receptors
(7,
15). Furthermore, recent cell culture studies have suggested that riluzole may also exert neurotrophic effects by stimulating the synthesis of brain-derived neurotrophic factor
(16). Although studies to ascertain if similar effects occur in vivo have not yet been undertaken, these findings are particularly noteworthy since conventional antidepressant treatments have been shown to increase the expression of brain-derived neurotrophic factor in the rat hippocampus and the infusion of intrahippocampal brain-derived neurotrophic factor produces antidepressant effects in preclinical behavioral models of depression
(17).
It is clear that our preliminary results need to be interpreted with caution given the small group size and the open-label nature of the study. Nevertheless, the significant and often striking response seen in some individuals who were refractory to traditional monoamine antidepressants suggests that direct modulation of the glutamatergic system may have considerable use for the treatment of mood disorders. Larger controlled studies are needed to confirm these preliminary findings.