Dysfunction of the prefrontal cortex in schizophrenia is associated with alterations in excitatory pyramidal neurons and in inhibitory neurotransmitter systems that regulate pyramidal neuron activity. For example, subjects with schizophrenia exhibit alterations in both pre- and postsynaptic markers of γ-aminobutyric acid (GABA) neurotransmission at the axon initial segment of pyramidal neurons in the prefrontal cortex, the site of action potential generation
(1,
2). It is interesting to note that the serotonin
1A (5-HT
1A) receptor has been reported to be highly concentrated at the axon initial segment of pyramidal neurons
(3), where it mediates neuronal hyperpolarization by activating potassium channels
(4). Postmortem autoradiographic studies have reported higher concentrations of 5-HT
1A receptors in the prefrontal cortex, including Brodmann’s area 46, in subjects with schizophrenia
(5), although a recent study that used positron emission tomography did not detect altered 5-HT
1A receptor binding in the dorsolateral prefrontal cortex
(6). However, these studies lacked the resolution required to identify 5-HT
1A receptors localized to the axon initial segment of pyramidal neurons. Consequently, in this study, we sought to determine whether the density or laminar distribution of axon initial segments with 5-HT
1A-like immunoreactivity is altered in the prefrontal cortex in subjects with schizophrenia and whether such changes are diagnostically specific.
Method
The 14 subject triads, each consisting of one subject with schizophrenia, one subject with major depressive disorder, and one comparison subject with no psychiatric disorder, examined in this study have been described previously
(2). The subjects within the triads were matched for sex and matched as closely as possible for age and postmortem interval. Subject groups did not significantly differ in mean age, postmortem interval, or tissue storage time
(2). After we obtained informed consent from the subjects’ next of kin, brain tissue was collected during autopsies conducted at the Allegheny County (Penn.) Coroner’s Office and handled in an identical fashion for each subject (i.e., 48-hour immersion fixation in a solution of 4% paraformaldehyde followed by cryoprotection and storage at –30° C)
(2). A consensus diagnosis was obtained for each subject by an independent panel of clinicians
(1).
Three tissue sections (40-μm thick) containing Brodmann’s area 46 of the prefrontal cortex from each subject were processed for immunocytochemistry
(1) in a randomized block design
(2) by using a rabbit polyclonal antibody (diluted to a concentration of 1:2000) raised against amino acids 170–186 in the second intracellular loop of the 5-HT
1A receptor
(7). Although the specificity of this antibody has been previously demonstrated
(7), the pattern of labeling differs from that of antibodies directed against the third intracellular loop and we cannot exclude cross-reactivity with an unknown protein localized to the axon initial segment. Sections were mounted on coded slides, and the reaction product was intensified with osmium tetroxide followed by silver nitrate
(8).
For each section, the axon initial segments with 5-HT
1A-like immunoreactivity were randomly sampled in the superficial (layers 2–3a) and deep (layers 5–6) zones of the cortex by using Stereo Investigator fractionator software (MicroBrightField, Inc., Williston, Vt.), as previously described
(1,
2). All quantification was conducted by one investigator (D.A.C.) who was blinded to the subject’s identifying number and diagnosis. Intrarater assessments of 5-HT
1A-labeled axon initial segment identification resulted in an intraclass correlation coefficient of 0.99 (95% confidence interval=0.98–0.99).
Between-group comparisons were made by using analysis of covariance (ANCOVA), with age, sex, postmortem interval, and tissue storage time as covariates. The influences of sex, lifetime history of substance abuse, cause of death, and psychotropic drug exposure at the time of death on group differences, relative to the comparison group, in the density of 5-HT1A-labeled axon initial segments were also assessed by using ANCOVA.
Results
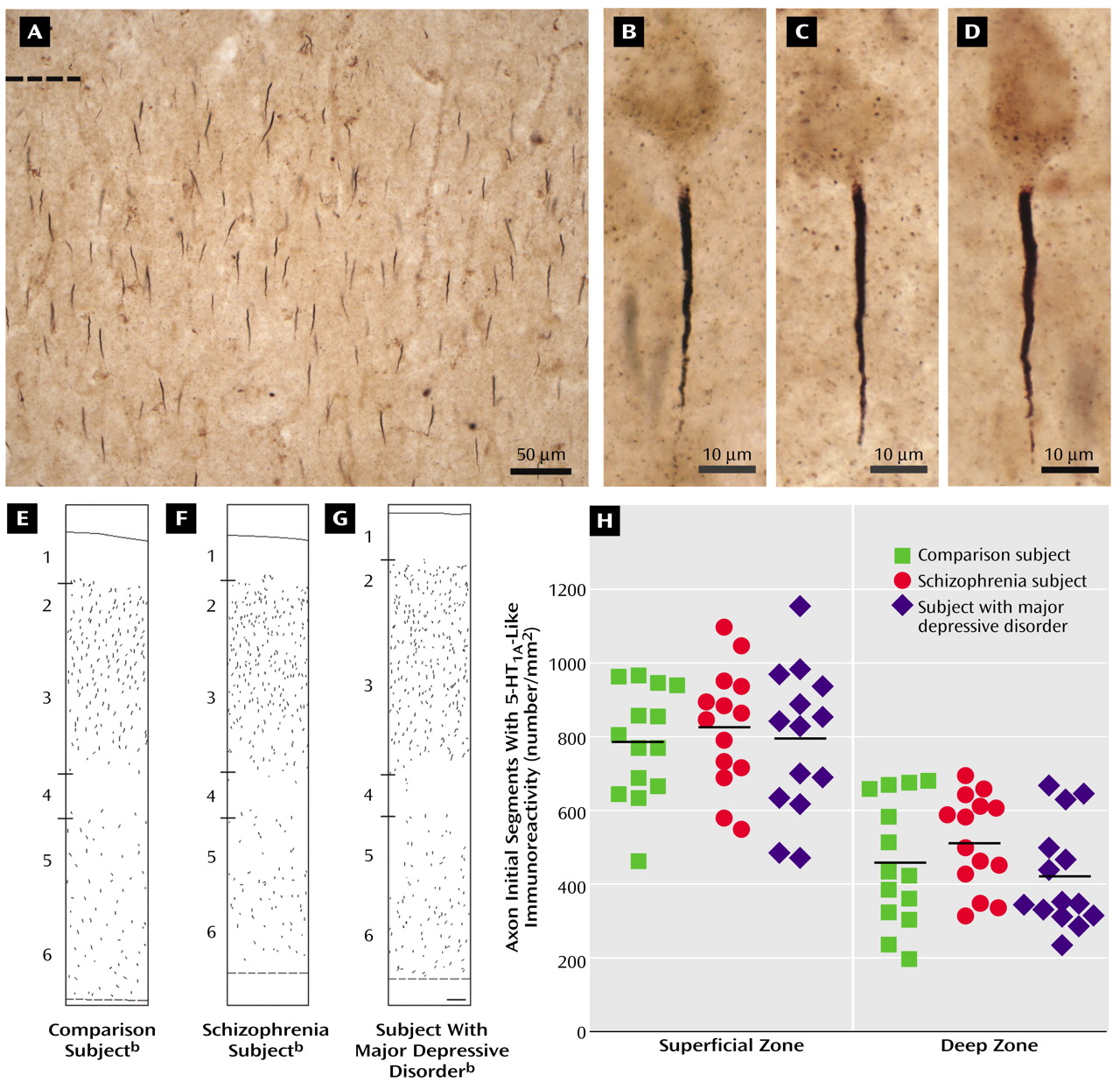
In brightfield photomicrographs, the 5-HT
1A-labeled axon initial segments appeared as intensely immunoreactive vertical structures that tapered from the superficial to deep cortical layers and were located below the unlabeled cell bodies of pyramidal neurons (
Figure 1). In all subjects, the density of the 5-HT
1A-labeled axon initial segments appeared to be greater in the superficial than in the deep cortical layers, and layer 4 contained few labeled axon initial segments (
Figure 1).
The mean density of 5-HT
1A-labeled axon initial segments (measured in segments/mm
2) did not differ between healthy comparison subjects, subjects with schizophrenia, and subjects with major depressive disorder in either the superficial zones (mean=784.1, SD=151.1; mean=827.2, SD=161.7; and mean=794.7, SD=197.6, respectively) or the deep zones (mean=456.9, SD=171.2; mean=515.0, SD=128.2; and mean=422.8, SD=143.4, respectively) of Brodmann’s area 46 (
Figure 1). In addition, the difference in 5-HT
1A-labeled axon initial segment density between individual subjects with schizophrenia or major depressive disorder and their matched comparison subjects did not differ in either the superficial or the deep cortical zones as a function of sex, lifetime history of substance abuse, cause of death, or psychotropic drug exposure at the time of death (F<0.84, df=1, 11, p>0.38).
Discussion
Due to the location of the axon initial segment near the site of action potential generation, inhibition at the axon initial segment plays a potent role in regulating the output of pyramidal neurons
(9). Convergent findings indicate a lower level of GABA neurotransmission at the axon initial segment in patients with schizophrenia, relative to comparison subjects with no psychiatric disorder. For example, in Brodmann’s area 46 in the prefrontal cortex of subjects with schizophrenia, relative to comparison subjects with no psychiatric disorder, immunoreactivity for the GABA membrane transporter is lower in the axon terminals of chandelier cells, the subclass of GABA neurons that selectively target the axon initial segment of pyramidal neurons, whereas greater immunoreactivity is found for the α2 subunit of the GABA
A receptor, which is preferentially localized to the pyramidal neuron axon initial segment
(1,
2). In contrast, the findings of this study demonstrate that in the same subject cohorts, neither the relative density nor laminar distribution of axon initial segments with 5-HT
1A-like immunoreactivity differed in the subjects with schizophrenia, relative to the comparison subjects with no psychiatric disorder or to the subjects with major depressive disorder. Because 5-HT
1A receptors at other cellular locations were not visualized with the antibody used in this study, changes in the overall density of these receptors cannot be excluded. However, consistent with the present findings, a previous study found no difference in the density of axons that were immunoreactive for the serotonin transporter in the prefrontal cortex of subjects with schizophrenia, relative to matched comparison subjects with no psychiatric disorder
(10).
Although the findings of this study were negative, they may be informative for the pathophysiology of schizophrenia. Specifically, studies in monkeys have revealed that adolescence is associated with marked changes in both pre- and postsynaptic markers of GABA neurotransmission at the pyramidal neuron axon initial segment
(11), whereas markers of 5-HT neurotransmission at the axon initial segment are stable during this developmental epoch
(12). In concert with the studies reviewed earlier, the current findings suggest that the maturation of GABA-mediated inhibition at the pyramidal neuron axon initial segment may be more relevant than 5-HT-mediated inhibition for the appearance of the clinical features of schizophrenia during late adolescence or early adulthood.