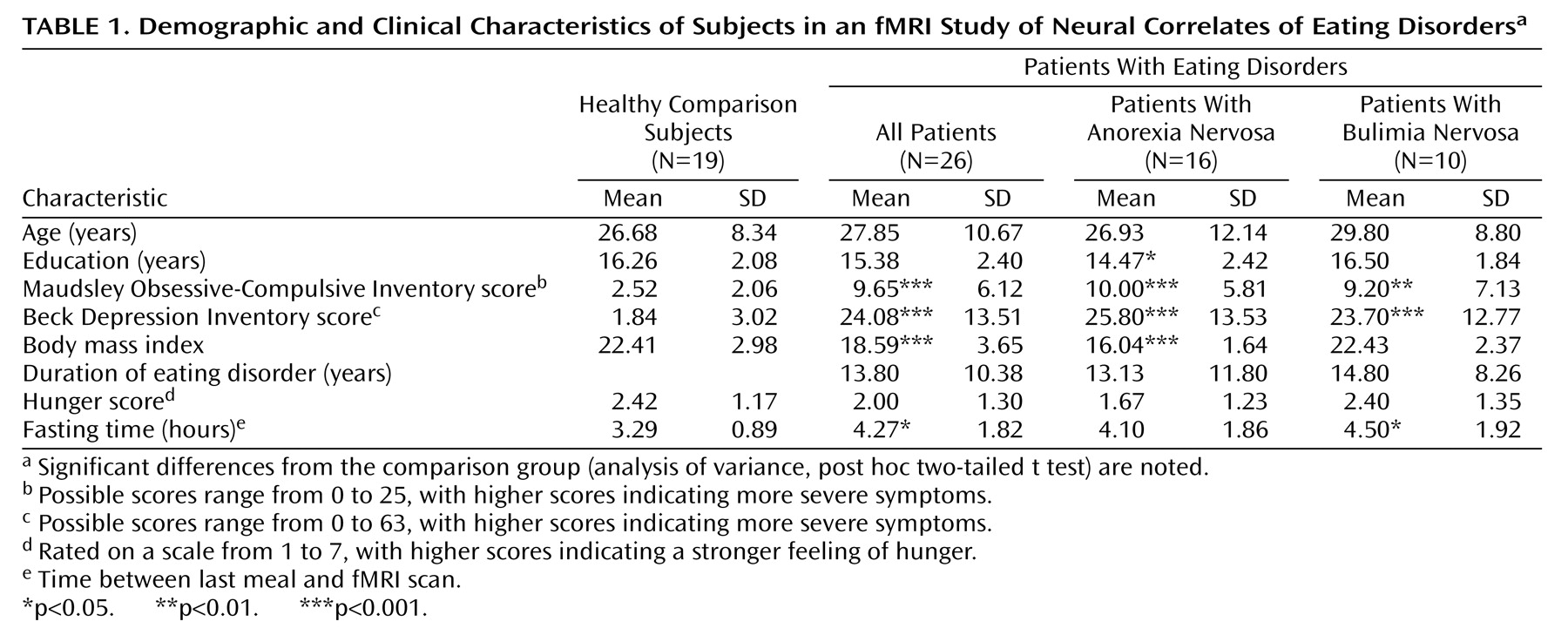
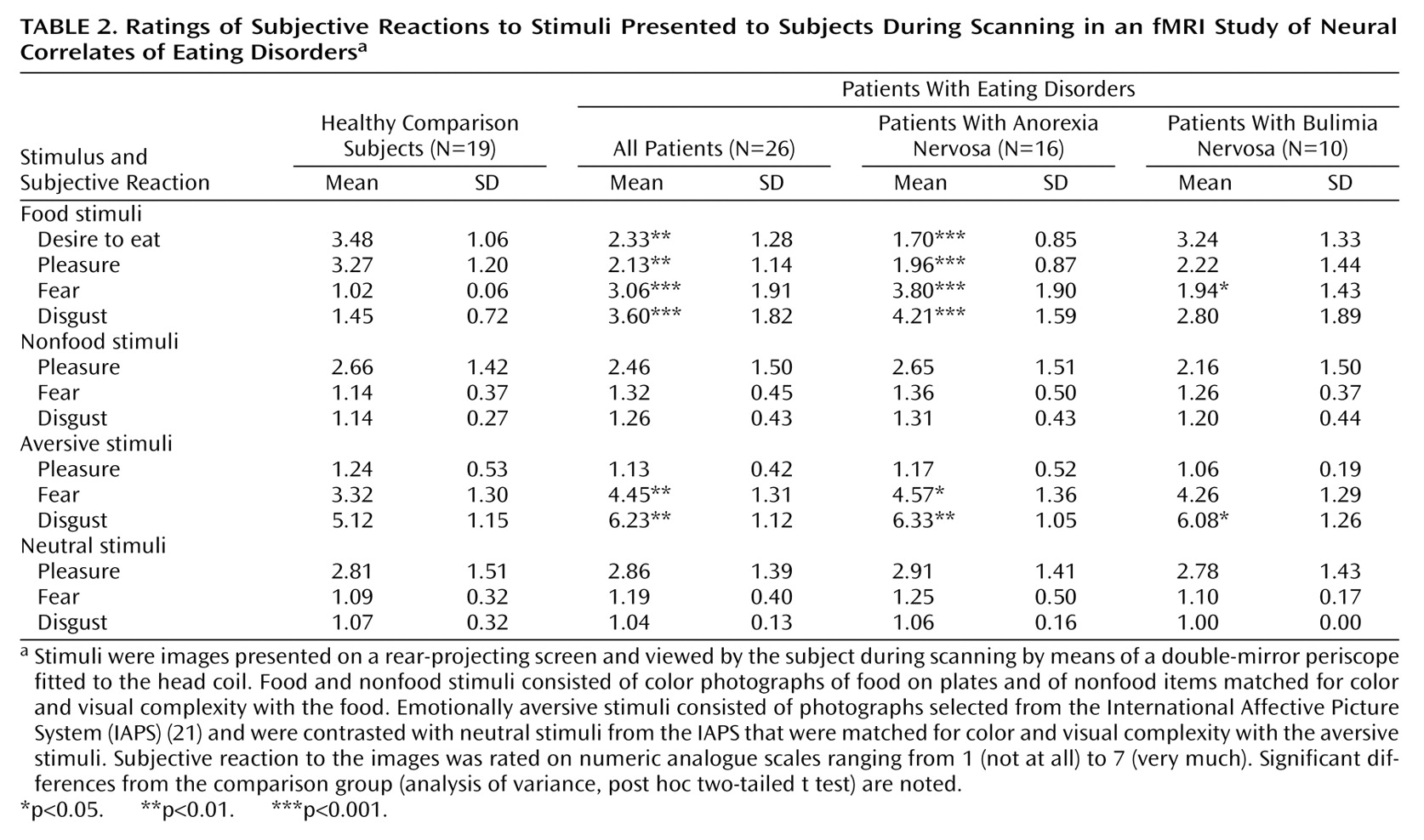
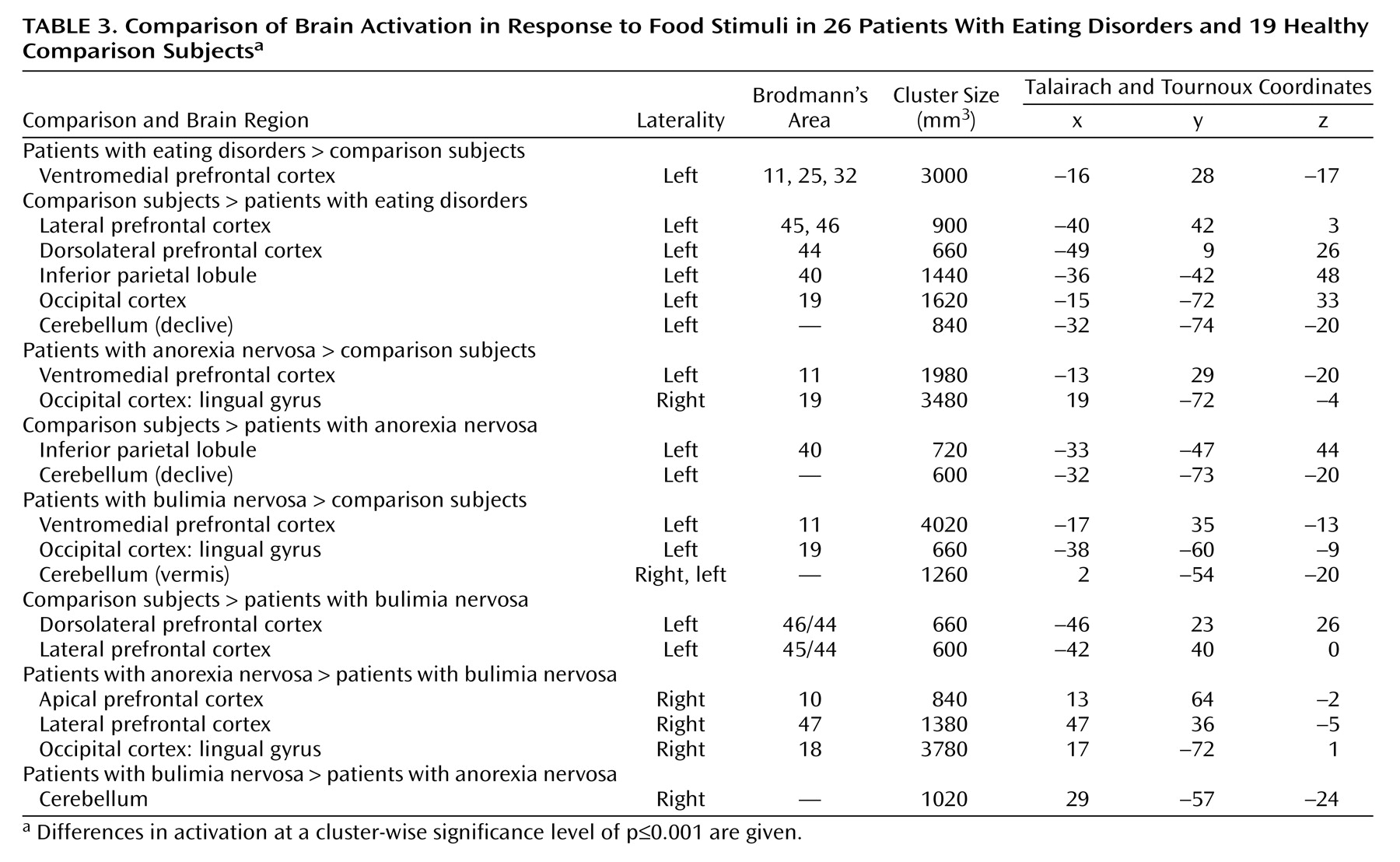
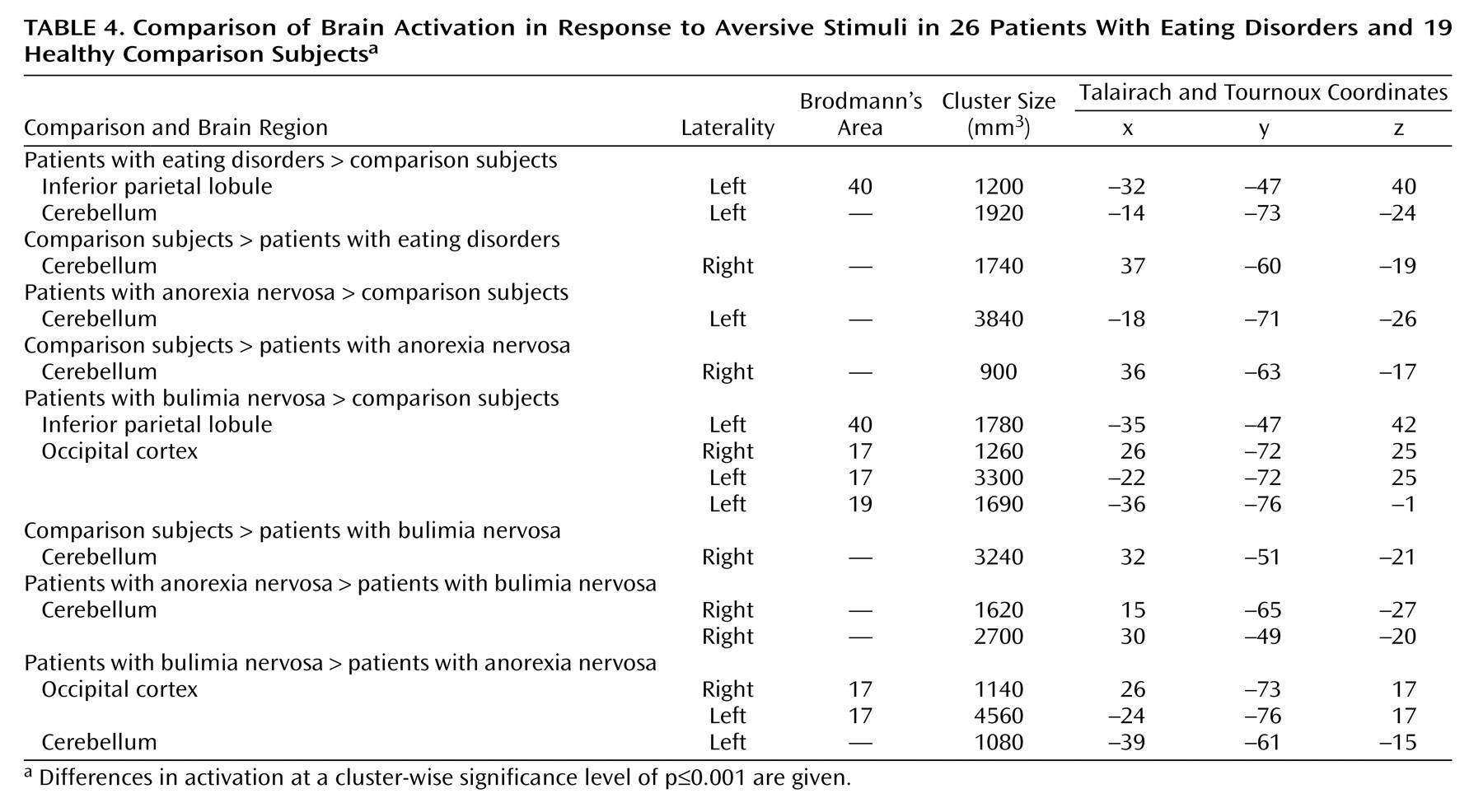
Evidence for a neural disturbance in eating disorders is compelling. The clinical syndrome can be reproduced by lesions in the right inferior prefrontal cortex
(4,
5). Association with perinatal complications is also suggestive of a cerebral cause
(6). Indirect evidence that eating disorders are neural diseases is inferred from neuropsychological, electrophysiological, neuropharmacological, structural neuroimaging, and functional neuroimaging investigations
(3).
Functional neuroimaging studies in eating disorders have either investigated brain function at rest or under controlled disorder-specific conditions. Resting-state experiments have found global cerebral hypofunction
(7), which is most pronounced in the anterior cingulate and frontal region
(8,
9) and in the parietal lobe
(7). In bulimia nervosa, parietal hypometabolism
(7) and a loss of the normal right > left asymmetry of brain glucose metabolism
(10) have been reported.
Controlled-condition experiments have used body shapes, eating, or food presentation to elicit symptom-related brain processes. In anorexia nervosa, perfusion of the frontal lobes (measured by single photon emission tomography [SPECT]) increased while subjects ate cake
(11), and, in a functional magnetic resonance (fMRI) experiment, viewing high-caloric drinks elicited responses in the prefrontal cortex, anterior cingulate, insular cortex, and amygdala
(12). A positron emission tomography (PET) study did not replicate these findings and reported food-related activity only in visual-associative cortices in seven anorexia nervosa patients who viewed high- versus low-caloric foods
(13). Amygdala response to morphed images of subjects’ bodies has been reported in three patients with anorexia nervosa
(14). Data on bulimia nervosa are limited. One SPECT study that included five patients with bulimia nervosa showed that high basal frontal perfusion decreased with eating, a response pattern opposite to that seen in anorexia nervosa
(15).
A summary of resting- and controlled-condition studies suggests that the same regions (prefrontal cortices, anterior cingulate) show diminished activity at rest but abnormally increased activity in response to food challenge. Some abnormalities (parietal hypometabolism at rest) are common to anorexia nervosa and bulimia nervosa
(7), while other (frontal reaction to eating) may differ between these diagnoses
(15). These similarities and differences add to the debate on whether eating disorders constitute one entity with a spectrum of manifestations or are several separate syndromes
(1,
2).
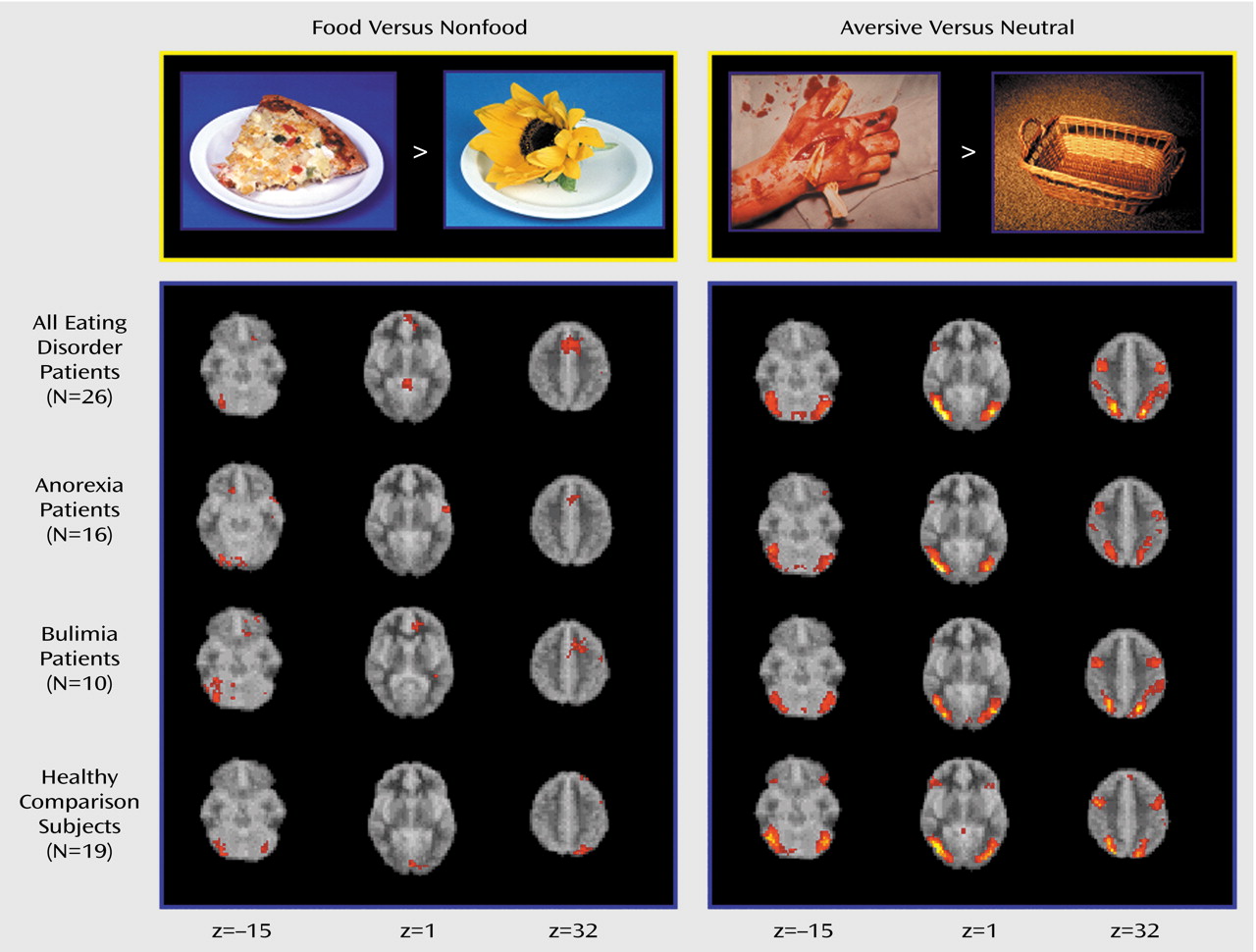
The propensity to specific attitudes (drive for thinness, fear of fatness) and behaviors (bingeing, restricting) is conceptualized as preferential activation of certain neural pathways and circuits. The functional relevance of neural circuits is inferred from activation patterns recorded in situations with a high probability of symptom manifestation. We used fMRI to investigate brain responses to food images in women with eating disorders and healthy female comparison subjects. Two general hypotheses were tested: 1) there will be activation patterns common to the whole eating disorders group, implying shared neural mechanisms, and 2) there will be responses specific to bulimia nervosa or anorexia nervosa, reflecting variations within the eating disorders spectrum. These differences should be specifically manifest in response to disease-specific material (food images), when symptom-related neural schemata are likely to be activated. To establish stimulus specificity, nonspecific emotionally salient stimuli were used in addition to food images.
Discussion
Exploration of cerebral activity during presentation of food images has identified activations common to the whole eating disorders group and specific to subgroups with particular eating disorder diagnoses. An abnormal prefrontal reaction was specifically manifested in response to food stimuli, whereas differences in cerebellar, occipital, and parietal activity were present in reaction to both emotional and food images.
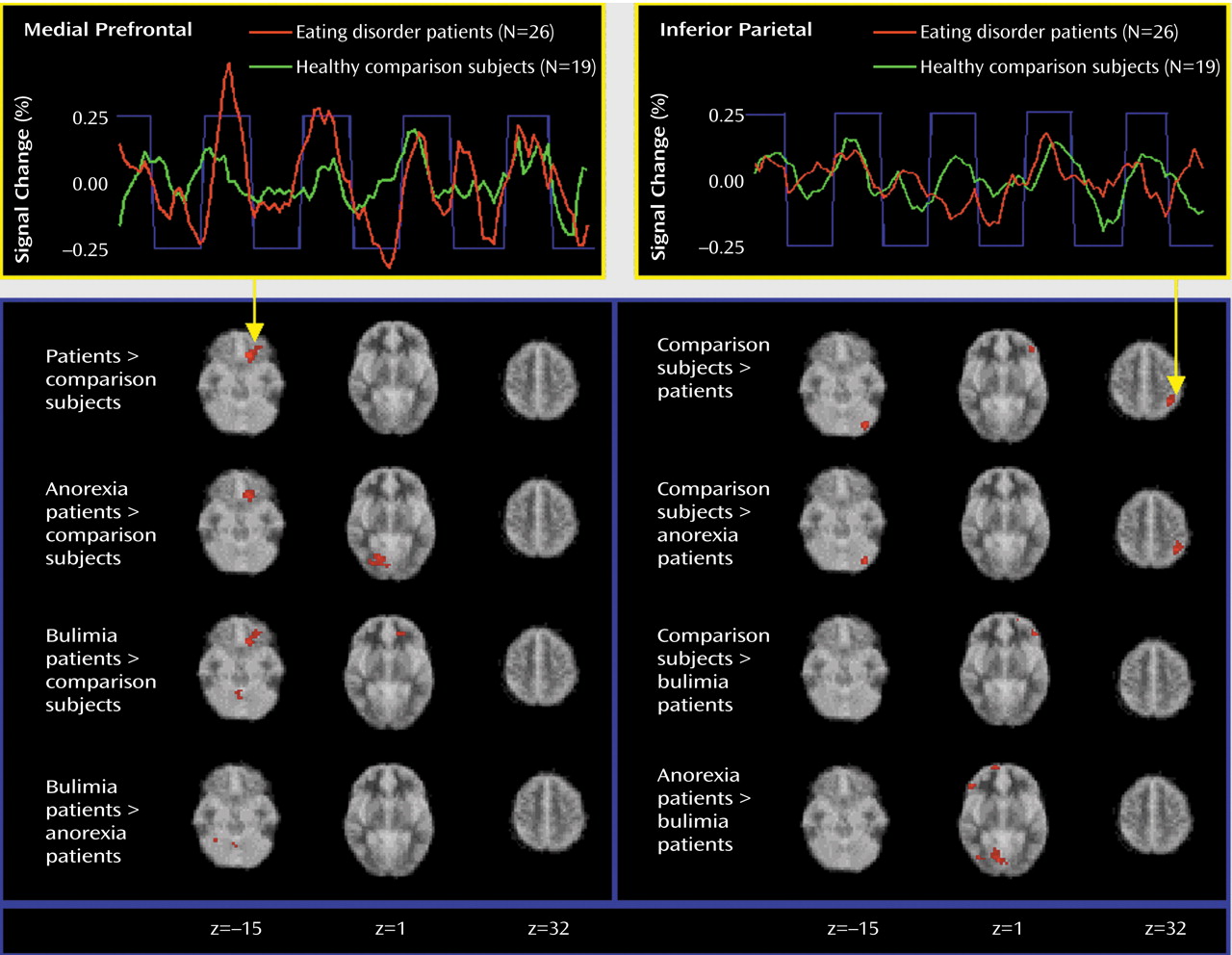
In response to food stimuli, patients with eating disorders recruited the medial orbitofrontal cortex and the anterior cingulate instead of the inferior parietal lobule and the left cerebellum, which were activated in the healthy comparison group. The finding of an increased medial prefrontal reaction to food was independently significant for both the anorexia nervosa and bulimia nervosa subgroups and therefore can be considered as a functional neural substrate common to these eating pathologies. The ventromedial prefrontal location of differential activity corresponds to locations where lesions are known to cause eating disorders–like syndromes
(4,
5) and to neuroimaging data
(8,
9,
11,
12,
15). The orbitofrontal and anterior cingulate cortices are a convergence zone for processing of emotional information and are consistently implicated in the pathogenesis of obsessive-compulsive and affective disorders
(25,
26). Because there is phenomenological overlap
(1,
17), comorbidity, and familial co-occurrence
(27) of these disorders with eating disorders, common neural mechanisms are suspected. Since addiction-like cue reactivity has been described in bulimia nervosa
(28), another parallel comes from studies in drug addiction, which show anterior cingulate and orbitofrontal activations related to craving and compulsive drug-taking behaviors
(16,
29). From these data, evidence converges on the anterior cingulate and the orbitofrontal cortex as major constituents in a dysfunctional network associated with compulsive and affective phenomena in a group of related disorders.
In our study, the patients with bulimia nervosa differed both from the patients with anorexia nervosa and from the comparison subjects by having decreased activation in the anterior and lateral prefrontal cortex in response to food. As the lateral prefrontal cortex is involved in suppressing undesirable behaviors
(30), diminished activity in this region may account for the loss of control in the eating behavior of bulimic patients. Lateral and apical prefrontal activity has been retrospectively associated with good outcome in anorexia nervosa
(20). In the future, frontal involvement in response to food may be evaluated as an outcome-modifying factor in bulimia nervosa.
Differences between groups in posterior cortical and cerebellar activation were present in reaction to both food and emotional stimuli. There are few data for comparison. Many neuroimaging studies have omitted the cerebellum or have used it as a baseline reference
(11,
15). In this study, diagnosis-specific changes in cerebellar activations have been highlighted. As these activations were present in both the food and aversive emotional stimuli conditions, they are likely to be associated with nonspecific processes, such as attention and arousal.
The present findings are largely compatible with previous reports. In detailed comparisons, however, there are two notable differences. First, activation in the amygdala differentiated anorexia nervosa subjects from comparison subjects in a previous study from our laboratory
(12), but in the present investigation there was no significant amygdala activation either in group maps or in between-group comparisons. Methodological differences, which might explain this difference in findings, include the type of stimuli (images of drinks labeled as high- or low-caloric were used previously) and the analytical procedures (more conservative in the current study). A more conclusive answer on the role of amygdala in eating disorders can be expected from studies with regionally optimized acquisition. Second, comparing anorexia nervosa and bulimia nervosa, we have found both common and differential components in frontal response to food stimuli, whereas an opposite pattern of frontal reaction to eating in anorexia nervosa and bulimia nervosa was reported by Nozoe et al.
(15). In comparing the two studies’ findings, different provocation paradigms (eating food versus seeing food) and neuroimaging methods (SPECT and fMRI) have to be taken into account. Most notably, Nozoe et al. chose to analyze regions of interest in only three of 35 axial slices; their findings may thus correspond to differential lateral prefrontal changes, while any common aspects of response in the ventromedial cortex may have been missed.
To our knowledge, the current study is the largest functional neuroimaging study in eating disorders to date and the first fMRI study in bulimia nervosa. A direct comparison of anorexia nervosa and bulimia nervosa patients allowed us to evaluate the similarities and differences of these groups. In addition to presentation of food images, a comparison paradigm was used to control for saliency and thus to differentiate between specific and nonspecific processes. Inclusion of subjects who were taking medication and of partially weight-recovered subjects was addressed in a confirmatory analysis by exclusion of the individuals with those characteristics.
Although the number of subjects in the current study is larger than in previous neuroimaging studies, it may not be large enough to explore the full eating disorders spectrum (e.g., subtypes of anorexia nervosa). The capacity of fMRI to detect signal changes is subject to regional differences in signal-to-noise ratio, and therefore the study procedures may be sensitive enough to detect differences in some brain regions but may lack power to do so in other regions (unpublished 2003 data of M. Brammer). Furthermore, fMRI explores paradigm-related changes in brain function but does not identify baseline differences, such as those reported in PET studies
(13). The paradigm-related changes of activity reported here will therefore be best appreciated as deviations from a baseline, which itself is altered in eating disorders
(7,
15).
The food paradigm was conceived as a symptom-provocation challenge, and the accompanying instruction was biased toward food as opposed to control stimuli. Therefore, the resulting activations cannot be interpreted as pure reactions to food. Because the same procedure was applied for all participants, the group comparisons were not affected by this instruction bias.
In conclusion, an abnormal focus of food-related activity in the medial prefrontal region has been identified in a large number of eating disorders patients. This activity was specifically manifested in response to symptom-related material and was independent of the type of eating disorders, use of medication, presence of depressive symptoms, fasting time, and body-weight status. Distinct aspects of anorexia and bulimia nervosa were reflected in the activity of circuits comprising the lateral and anterior prefrontal cortices. The similarity of findings in eating, obsessive-compulsive, and affective disorders and in addiction raises the possibility of a common compulsion- and affect-related circuit that is based in the medial prefrontal cortex and activated by disease-specific stimuli in these various conditions.