Late-life depression is associated with structural abnormalities of cortical
(1–
3) and subcortical
(4) regions as well as greater severity of white matter hyperintense lesions
(3,
5,
6). In addition, functional imaging studies in the elderly have demonstrated metabolic changes in frontal regions
(7,
8). One theory linking these findings is that white matter lesions disrupt the neural circuits involved in mood regulation, resulting in impaired cortical and subcortical function, which contributes to the pathogenesis of geriatric depression. This study tested for microstructural abnormalities in one such neural pathway, the dorsolateral prefrontal circuit.
The dorsolateral prefrontal circuit modulates mood regulation and cognitive ability. The circuit originates in the dorsolateral prefrontal cortex (Brodmann’s areas 9 and 10), projects to the dorsolateral head of the caudate nucleus, then continues to the lateral dorsomedial globus pallidus and, finally, to the ventral anterior and mediodorsal thalamus. The mediodorsal thalamus then sends fibers back to the dorsolateral prefrontal cortex
(9). Injury to this circuit is associated with executive dysfunction
(9,
10), which is also seen in depressed elderly subjects
(11,
12).
We used diffusion tensor imaging to examine the microstructure of dorsolateral prefrontal cortex white matter. We hypothesized that elderly depressed subjects would exhibit greater microstructural abnormalities (measured as lower fractional anisotropy) in the dorsolateral prefrontal cortex than elderly comparison subjects who were not depressed.
Method
All subjects were participants in the NIMH-sponsored Conte Center for the Study of the Neuroscience of Depression at Duke University. Depressed subjects were 60 years or older, had scores ≥16 on the Center for Epidemiologic Studies Depression Scale
(13), or fulfilled DSM-IV criteria for major depression. Exclusion criteria included 1) another major psychiatric illness, including substance abuse or dependence; 2) primary neurological illness, including dementia or stroke; 3) medical illness impairing cognitive function; and 4) indwelling metal, precluding magnetic resonance imaging (MRI).
Comparison subjects were recruited from the Aging Center Subject Registry at Duke University, a listing of community-dwelling elderly individuals willing to participate in aging research. Eligible comparison subjects had a nonfocal neurological examination, no self-report of neurological or depressive illness, and no evidence of depression based on the NIMH Diagnostic Interview Schedule
(14).
Study purposes and procedures were explained to each participant. Those providing written informed consent were enrolled. The Duke University Institutional Review Board approved this study.
All subjects answered questions regarding the presence of heart disease (phrased as “heart trouble”) and hypertension; a format developed for the NIMH Epidemiological Catchment Area Study
(15) was used for these questions. Depressed subjects additionally reported their age at first onset of depression, and the severity of their depression was measured with the Montgomery-Åsberg Depression Rating Scale
(16).
Subjects were screened for contraindications to MRI. Axial images were acquired with a Signa 1.5-T MR system (GE Medical Systems, Milwaukee) using the standard head (volumetric) radiofrequency coil. After localization of slices to be imaged, the automated manufacturer algorithm adjusted the shim currents to obtain good magnetic field uniformity.
The diffusion images were acquired with a two-dimensional echo-planar pulse sequence (TE=109 msec and TR=12000 msec) that acquired an image in each of six diffusion weighting directions with a b value of 1000 seconds/mm
2 as well as one additional image with a b value of zero. The direction scheme followed that of Basser and Pierpaoli
(17). The other parameters for the sequence were field of view=24×24 cm, 90 KHz bandwidth; 1 excitation; 128×128 matrix; 5-mm slice thickness; and slice gap=2.5 mm. Diffusion weighted images were processed by using custom MATLAB (Mathworks, Natick, Mass.) scripts that calculated
(18,
19) the diffusion tensor eigenvalues in each voxel. A diffusion fractional anisotropy image
(18) was then calculated.
Fractional anisotropy images were processed on SUN workstations with Analyze 4.0 (Mayo Clinic, Rochester, Minn.). A reader blind to the subjects’ depression status placed oval regions of interest in the left occipital lobe and bilaterally in the white matter of the superior and middle frontal gyri; gray matter was avoided. The left occipital lobe was a control region not expected to exhibit changes associated with depression. The frontal gyri regions of interest were 55 mm2 in size, and the occipital region of interest was 78.2 mm2. The frontal gyri regions of interest were placed on the most caudad slice where both gyri were visible as separate structures and halfway between the anterior boundary of the brain and the precentral sulcus, which defined the posterior boundary. The left occipital lobe region of interest was placed on the most caudad slice on which the anterior horns of the lateral ventricles were still visible and was positioned just posterior to the lateral ventricle. Dorsolateral prefrontal cortex regions of interest were placed while avoiding any visible lesions; occipital region of interest placement was made independent of the presence or absence of lesions.
Reliability was established by repeated measurements on multiple magnetic resonance diffusion tensor imaging scans. Intraclass correlation coefficients (ICCs) attained were as follows: left superior frontal gyrus, ICC=0.86; right superior frontal gyrus, ICC=0.95; left middle frontal gyrus, ICC=0.98; right middle frontal gyrus, ICC=0.94; and left occipital ICC=0.74. Student’s t tests were used to determine differences between mean fractional anisotropy values for each region. Logistic regression models confirmed differences while accounting for age, gender, and comorbid medical disorders.
Results
The study group included 16 comparison and 17 depressed subjects. The mean Montgomery-Åsberg Depression Rating Scale score of the depressed subjects was 23.06 (SD=4.34); their mean age at onset of depression was 41.65 years (SD=24.02). There was no significant difference in age between comparison subjects (mean=69.75, SD=6.38) and depressed subjects (mean=67.53, SD=6.12) (t test estimate=1.02, df=31, p=0.32). There were fewer men in the comparison group (two of 16) than the depressed group (seven of 17), but this did not reach a level of statistical significance (p=0.12, Fisher’s exact test, two-tailed). There was also no significant difference between groups in those reporting a diagnosis of hypertension (five comparison and four depressed subjects) (χ2=0.25, df=31, p=0.62) or heart disease (three comparison and two depressed subjects) (χ2=0.31, df=31, p=0.58).
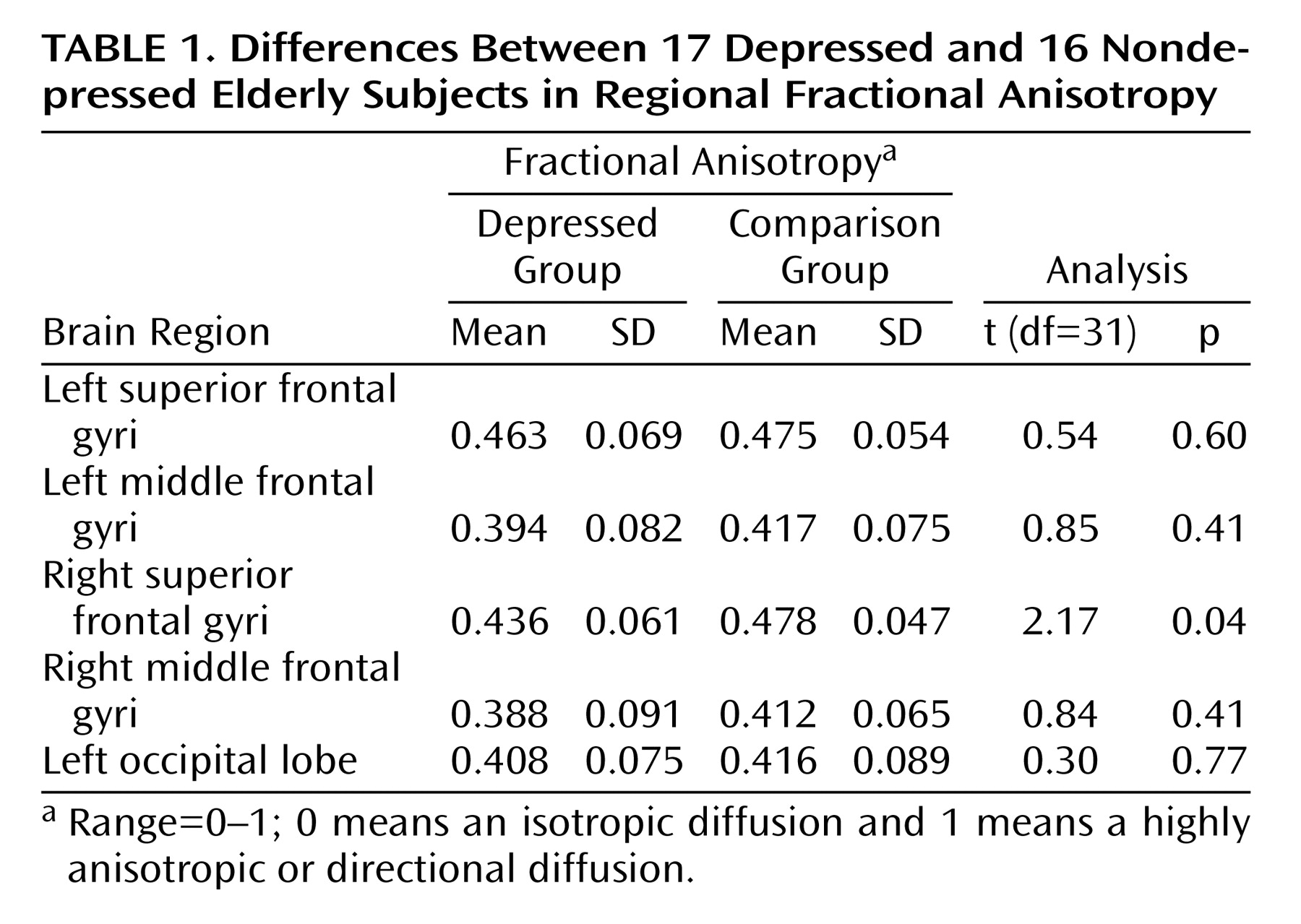
Depressed subjects exhibited lower fractional anisotropy values than did comparison subjects in all regions of interest (
Table 1). This difference reached a level of statistical significance only in the right superior frontal gyrus. This difference in fractional anisotropy between subject groups remained significant in a logistic regression model (estimate=0.025, t=2.39, df=31, p=0.03), even when we controlled for age (estimate=–0.0004, t=–0.23, df=31, p=0.82), gender (estimate=0.013, t=1.07, p=0.30), and presence of hypertension (estimate=0.010, t=0.90, p=0.38) and heart disease (estimate=–0.006, t=–0.41, df=31, p=0.69).
Discussion
The principal finding of this study is that elderly depressed individuals exhibit lower fractional anisotropy in the white matter of the right superior frontal gyrus than do nondepressed elderly subjects. Despite a relatively small number of study subjects, this finding is robust and independent of age, gender, or report of hypertension or heart disease. To our knowledge, this is the first study to associate specific white matter microstructural changes with a diagnosis of depression, although others have associated microstructural changes lateral to the anterior cingulate with depression outcomes
(20).
Our findings support the theory that microstructural changes in white matter may result in disconnection of cortical and subcortical regions. If these changes affect circuits involved in mood regulation, it may contribute to the pathogenesis of depression. Impairment of the dorsolateral prefrontal cortex may result in a dorsolateral prefrontal syndrome characterized by executive dysfunction
(9,
10). Executive dysfunction has also been found in depressed elderly subjects
(11,
12) and is associated with poorer depression outcomes
(21,
22).
The dorsolateral prefrontal cortex is also involved in mood regulation. Hypometabolism of the right dorsolateral prefrontal cortex has been repeatedly described in positron emission tomography studies of depressed subjects
(23,
24) and in subjects experiencing transient sadness
(25), although this finding is not universal
(26). Further, this region exhibits increases in metabolism as depressed individuals recover
(25). Suppression of sadness is also associated with greater activation of the right dorsolateral prefrontal cortex
(27).
The principal limitation of this study is the lack of specificity of our findings for the dorsolateral prefrontal circuit. Not all fibers within the white matter of the right superior frontal gyrus project to the dorsolateral caudate and lateral orbitofrontal cortex. The exact determination of the fraction of white matter in this gyrus that is part of the dorsolateral prefrontal circuit must await more specific forms of analysis, such as fiber tractography based on diffusion tensor imaging. Other limitations include the small number of subjects and dependence on self-report for measures of vascular disease. Additionally, our measures of heart disease likely represent a heterogeneous group of cardiac disorders. Our not finding an association between anisotropy and cerebrovascular risk factors should thus be viewed cautiously given postmortem studies that found greater expression of markers of ischemia-induced inflammation in the dorsolateral prefrontal cortex of depressed subjects
(28).
Our study supports theories of neural circuitry involved in depression. Larger studies are needed, particularly to examine the relationship between these abnormalities and depression severity, response to treatment, or risk of relapse. Such studies may contribute to the development of antidepressant agents that target abnormalities in frontostriatal function.