Pediatric bipolar disorder is a serious mental illness with significant psychosocial dysfunction
(1). It often presents with a mixed or “dysphoric” picture characterized by frequent short periods of intense mood lability and irritability, rather than classic euphoric mania
(2,
3). Mood symptoms are more likely to be mixed, to be continuously present, or to demonstrate rapid cycling
(4). In adults, these symptom patterns are especially responsive to divalproex sodium
(5).
A 2-year longitudinal, naturalistic follow-up study of pediatric bipolar disorder found the condition to be poorly responsive to treatment
(6), although more than half of the patients in this study did not receive a mood stabilizer at any time during this study. A few small open studies have suggested that divalproex sodium is an effective treatment for mania
(7–
9).
Kowatch and colleagues
(10) randomly assigned 42 pediatric outpatients with bipolar I or bipolar II disorder during mixed or manic episodes to 6–8 weeks of open treatment with lithium, valproate, or carbamazepine. Response rates were 38% for carbamazepine, 38% for lithium, and 53% for sodium divalproex; the criterion for response was a ≥50% change from baseline to exit in the Young Mania Rating Scale
(11) score (χ
2=0.85, df=2, p=0.60). Each of the three mood stabilizers was well tolerated, and no serious adverse effects were seen. Thus, the few studies available suggest both efficacy and safety for divalproex in the acute treatment of manic and mixed states of pediatric bipolar disorder.
Attention deficit hyperactivity disorder (ADHD) co-occurs with pediatric bipolar disorder in 29%–98% of patients
(1,
3,
12–15). ADHD may worsen the prognosis or complicate the treatment of bipolar disorder. Adolescents with manic symptoms who have a history of early childhood ADHD may have a poor response to lithium
(16).
ADHD often causes significant school dysfunction and warrants treatment. Stimulants are the agents of choice for ADHD that is uncomplicated by bipolar disorder
(17). Alternative treatments include antidepressants that often worsen mood symptoms, increase cycling, or precipitate mania. Whether mood stabilizers alone will improve ADHD symptoms has not been evaluated, although most clinicians believe that mood stabilizers are ineffective, because symptoms of inattention, hyperactivity, and impulsivity seem to persist even after the mood symptoms have been stabilized.
To our knowledge, there are no prospective randomized, controlled trials of stimulant treatment of ADHD concurrent with pediatric bipolar disorder. One chart review evaluated the adjunctive use of stimulants for concurrent ADHD in pediatric patients with bipolar disorder
(18). The researchers found ADHD improvement to be 7.5 times greater with tricyclic antidepressants when mania was controlled with various mood stabilizers than when mania was not controlled, although the degree of ADHD improvement in these 38 patients was only modest, compared to findings in studies of uncomplicated ADHD. Furthermore, tricyclic antidepressant use was associated with manic symptom relapse in 76% of patients. In addition, two small studies suggested that stimulants may be effective for ADHD in pediatric patients with bipolar disorder
(19,
20).
Stimulant treatment of ADHD in the context of bipolar disorder raises the possibility of the worsening of mania or the induction of cycling. Clinical observation and case reports suggest that some children who ultimately receive a diagnosis of bipolar disorder will experience a worsening of mood symptoms or the onset of mania when treated with psychostimulants
(21,
22). Because of clinical concerns about use of stimulants for ADHD, some clinicians and patient advocacy groups have recommended against their use in youths with bipolar disorder. DelBello and colleagues
(23) reported that adolescents with bipolar disorder had a significantly earlier onset of manic symptoms if they had been previously treated with a psychostimulant, although another report found no relation between early methylphenidate treatment and bipolar disorder symptoms
(24).
To summarize, there are no prospective reports on the efficacy of divalproex sodium or other mood stabilizers alone for the treatment of ADHD in the context of pediatric bipolar disorder and no controlled trials of an adjunctive stimulant for concurrent ADHD symptoms in patients with pediatric bipolar disorder whose condition has been stabilized through treatment with a mood stabilizer.
To address these issues, we conducted a randomized, double-blind, placebo-controlled study of mixed amphetamine salts (Adderall) to treat ADHD symptoms after mood stabilization with divalproex sodium in pediatric patients with bipolar disorder and ADHD. The study had two objectives: 1) to determine if divalproex sodium monotherapy was beneficial for ADHD and mania in an open 8-week trial and 2) to determine if mixed amphetamine salts was safe and effective, compared to placebo, for ADHD symptoms after control of manic symptoms through the use of divalproex sodium.
Method
The study began with 8 weeks of open treatment with divalproex sodium. To enter the subsequent 4-week, double-blind, placebo-controlled crossover trial of mixed amphetamine salts versus placebo, patients were required to have experienced significant reduction in the symptoms of mania (≥50% decrease in Young Mania Rating Scale scores from baseline) with divalproex sodium treatment. After the double-blind phase, patients could be treated openly with divalproex sodium and mixed amphetamine salts during the 12-week follow-up phase. Clinical evaluations were conducted monthly.
Subjects
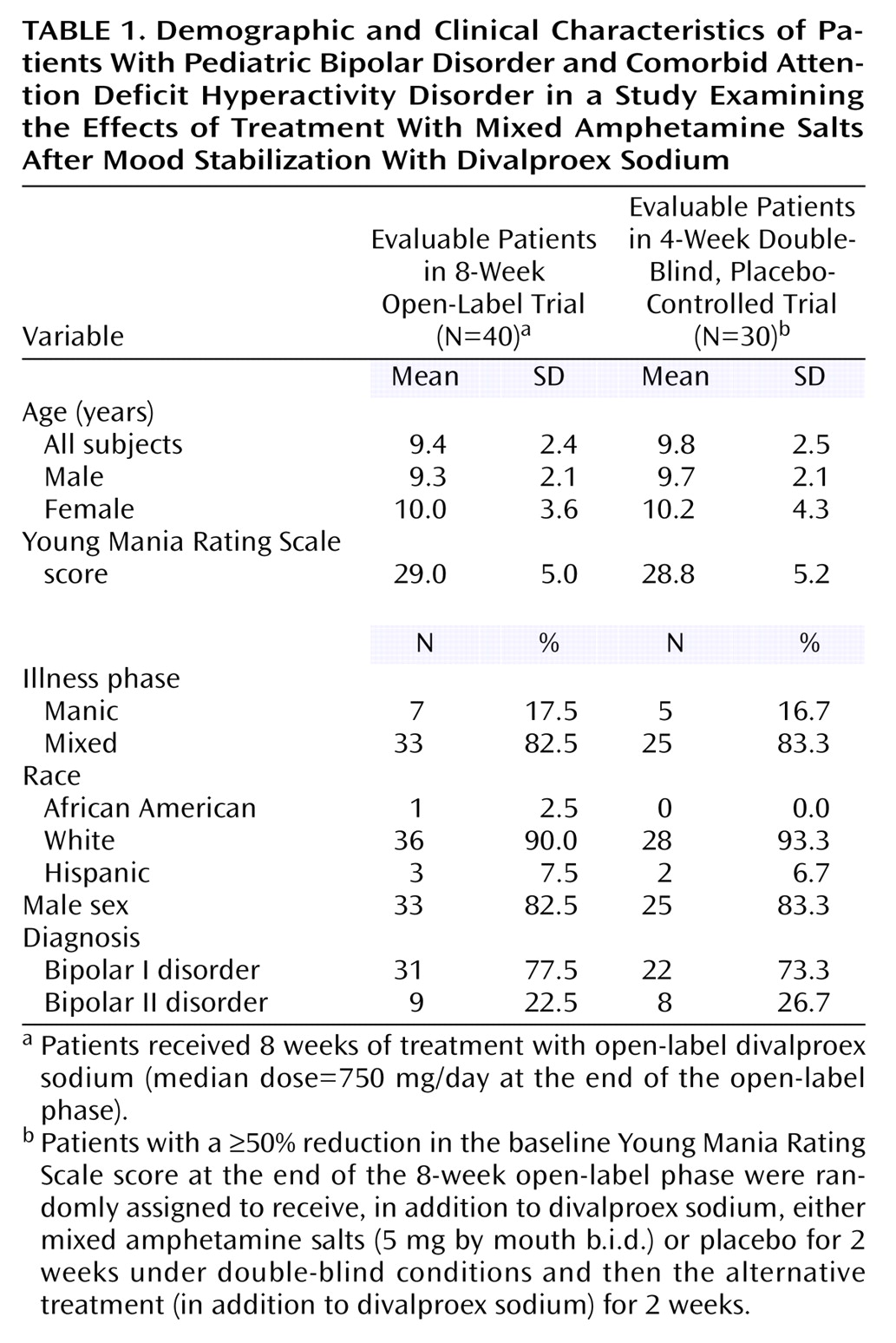
The study was approved by the University of Texas Southwestern Medical Center Institutional Review Board. All subjects provided written informed assent, and parents provided written informed consent at the initiation of the study before baseline evaluation. Study subjects were recruited from a university-based outpatient pediatric psychiatry clinic and the community. Eligible subjects were males and females, 6–17 years of age, who met the DSM-IV criteria for both bipolar I or bipolar II disorder (in either the mixed, manic, or hypomanic phase) and ADHD. All subjects had to score ≥14 on the Young Mania Rating Scale at baseline, to have scores exceeding two standard deviations from normal on the hyperactivity index of the Conners’ Teachers and Parents Rating Scales
(25), and to be of normal intelligence (IQ>70) on the basis of clinical impression or formal testing.
Patients with current or lifetime diagnoses of schizophrenia, schizoaffective disorder, eating disorder, autism or other pervasive developmental disorder, obsessive-compulsive disorder, or a mood disorder due to a general medical condition were excluded, as were those with current psychotic symptoms and those in concurrent psychotherapy. Patients who posed a serious suicide risk, those using any psychotropic medication within 2 weeks before the baseline evaluation (4 weeks for fluoxetine), those with a history of substance abuse or dependence within the past 3 months, those with any illicit drug use in the last 3 weeks, and menstruating girls who were not using a reliable form of contraception were also excluded. Finally, patients were excluded if they had a history of lack of efficacy in a trial of divalproex sodium that lasted at least 3 weeks and in which valproic acid serum levels were between 75–125 μg/ml.
Measures
Psychiatric diagnoses were made by using DSM-IV criteria in a clinical interview conducted by an experienced trained research clinician. Clinical diagnoses were confirmed by using a structured clinical interview (Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia
[26]). This interview was administered to a parent (usually the mother) and the subject by trained research assistants and research nurses. All research personnel were trained by the research group at Washington University. Results of the interviews were reviewed by the research physician as an additional quality control measure. Diagnostic criteria for mania required the presence of either elation or grandiosity in order to assist in the differentiation of mania from ADHD and other behavioral problems.
General medical evaluation included a medical history, review of systems, a physical examination, and a brief laboratory test battery (measurement of transaminases, complete blood count, urine drug screen, urine pregnancy test). The evaluation of transaminase, complete blood count, and valproic acid levels was repeated monthly throughout the study for safety reasons.
Outcome Assessments
The Young Mania Rating Scale
(11), an 11-item clinician scale, was used to assess the severity of manic symptoms. The Young Mania Rating Scale has been established as a valid and reliable instrument for rating of clinical symptoms of mania in children and adolescents
(27).
The Clinical Global Impression (CGI) improvement subscale
(28) was selected as the main outcome measure for ADHD symptoms. This scale was adapted to allow the clinician to specifically assess the symptoms of ADHD. The CGI improvement ratings were based on clinical interview of the subject and parent, direct observation of the subjects, and information obtained from the Conners’ Teachers and Parents Rating Scales. The CGI improvement scores range from 1 (very much improved) to 4 (no change) to 7 (very much worsened). The CGI improvement score for ADHD was obtained for the initial 8-week (divalproex sodium alone) phase by using baseline ADHD symptom severity scores as the benchmark against which change was measured. For the double-blind crossover trial, ADHD status at the initiation of this phase was the basis for subsequent CGI improvement ratings.
Both the Young Mania Rating Scale and CGI improvement subscale were administered by doctoral-level clinicians trained in their use for research. The intraclass correlations for the instruments were 0.84 for the Young Mania Rating Scale and 0.92 for the CGI improvement subscale.
Data on medication side effects were collected by using the Side Effects Form for Children and Adolescents
(29). The form was completed by the research assistant and reviewed by the clinician. Valproic acid trough levels were measured 12 hours (plus or minus 1 hour) after the last divalproex sodium dose at a certified clinical laboratory.
Treatment
Participants began the study with a 2-week evaluation period that included psychotropic medication washout if necessary. Thereafter, an 8-week open trial of divalproex sodium was conducted during which subjects were seen weekly and assessed with the CGI improvement and severity subscales, Young Mania Rating Scale, and Side Effects Form for Children and Adolescents. Subjects with a ≥50% decrease in the baseline Young Mania Rating Scale score at week 8 were considered divalproex sodium responders.
Divalproex sodium responders were eligible for random assignment to a 2-week double-blind trial in which they received either mixed amphetamine salts (5 mg by mouth b.i.d.) or placebo. At the conclusion of this initial 2-week trial, patients were crossed over to the alternative condition under double-blind conditions. Divalproex sodium dosing was held steady during this 4-week crossover trial. Minor increases (125 mg/day) were made for two patients in the follow-up phase, during which patients received both mixed amphetamine salts and divalproex sodium (open label) for another 12 weeks with monthly assessments.
Statistical Methods
Two-sample t test or chi-square analyses were used to compare baseline features of individuals included in the randomized crossover trial (N=30) and those who were not included (N=10).
The longitudinal Young Mania Rating Scale and CGI improvement score for ADHD observations during the 8-week open-label divalproex sodium phase were analyzed by using a random regression model
(30) to assess the significance of change over time. Young Mania Rating Scale and CGI improvement scores from the crossover trial were analyzed in two stages. In the first stage, it was determined that there was no significant carryover effect (i.e., there was no difference in drug effect between those who received mixed amphetamine salts and then placebo and those who received placebo and then mixed amphetamine salts). The potential for a carryover effect was tested by a drug- (mixed amphetamine salts versus placebo) by-time interaction in a random regression analysis. In the second stage, the difference between mixed amphetamine salts and placebo (irrespective of the order of administration) was tested by a paired t test. Testing was implemented with SAS software (SAS Institute, Inc., Cary, N.C.).
Results
Table 1 presents the clinical and demographic characteristics of the subjects. For the overall group of 40 subjects, the average ages of the boys and girls were not different (girls: mean=10.0 years, SD=3.6; boys: mean=9.3 years, SD=2.1) (t=0.69, df=38, p=0.50). The 30 patients included in the crossover trial and the 10 patients not included in the crossover trial did not differ by any baseline feature.
Open-Label Divalproex Sodium
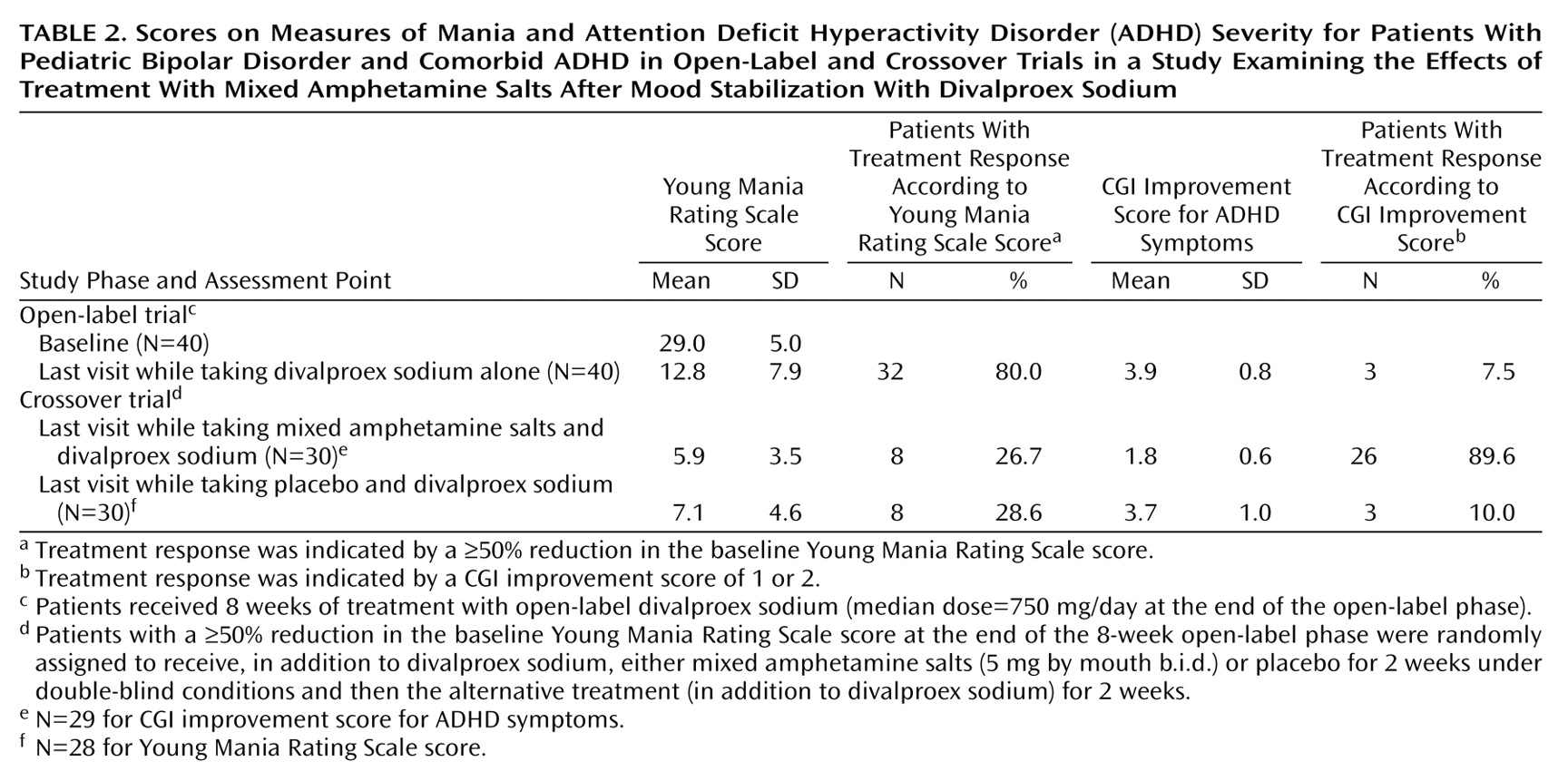
Random regression analysis showed that the subjects’ Young Mania Rating Scale scores decreased significantly during the open-label phase (2.2 points per week, F=276.6, df=1, 122, p<0.0001). Thirty-two (80%) of 40 initial subjects treated with divalproex sodium achieved a significant manic symptom response (≥50% decrease in baseline Young Mania Rating Scale scores) by the last open-label phase visit.
CGI improvement scores for ADHD showed a nonsignificant decrease of 0.001 points per week during the open-label phase (F=0.0025, df=1, 150, p=0.96). It is noteworthy that 7.5% of the subjects (three of 40 subjects) had a CGI improvement score of 1 or 2 (very much improved or much improved) at the last open-label phase visit, 12.5% (five of 40 subjects) had a CGI improvement score of 3 (mild improvement), 65.0% (26 of 40 subjects) had a score of 4 (no change), and 15.0% (six of 40 subjects) had a score of 5 (minimally worse). Only one subject had a score below the cutoff to declare ADHD present (two standard deviations from normal on the hyperactivity index of the Conners’ Teachers and Parents Rating Scales).
Altogether, 32 (80.0%) of 40 subjects achieved response on the Young Mania Rating Scale by exit from the open-label phase, while three (7.5%) of 40 subjects achieved an ADHD response (a CGI improvement score of 1 or 2) at exit. One subject (2.5%) was a responder according to both the Young Mania Rating Scale and CGI improvement scores. No statistically significant association between mania response (Young Mania Rating Scale) and ADHD response (CGI improvement) was observed (p=0.10, Fisher’s exact test).
Mixed Amphetamine Salts Versus Placebo
Thirty-one patients were randomly assigned to the crossover trial comparing mixed amphetamine salts and placebo, but only 30 provided postrandomization data. One subject’s parent unblinded herself by removing the overencapsulation on the mixed amphetamine salts/placebo in the initial assignment. We therefore did not have evaluable data for this patient. Among the 30 subjects with at least one postrandomization visit, one parent declined random rerandomization because she believed that her child received mixed amphetamine salts initially.
Of the nine subjects who were not randomly assigned in the crossover trial comparing mixed amphetamine salts and placebo, three had worsening of manic symptoms in the open-label divalproex sodium phase of the study and were hospitalized. Four of the nine did not respond to divalproex sodium monotherapy, withdrew from the study, and received additional open treatment. One family moved out of the area abruptly and was withdrawn from the study. Finally, one patient no longer met the study entry criterion of the Conners’ Teachers and Parents Rating Scales hyperactivity index score at the end of the 8-week open-label divalproex sodium treatment phase.
As for ADHD symptoms, the subjects who were randomly assigned to receive mixed amphetamine salts (N=14) in the first phase of the crossover trial had a CGI improvement score of 1.7 (SD=0.6) at the end of the first crossover phase, and the subjects who received placebo (N=16) had a CGI improvement score of 3.4 (SD=1.0). The difference between groups was significant (t=–5.8, df=28, p<0.0001). The potential for a carryover effect from phase 1 to phase 2 of the crossover trial was tested with an interaction term (treatment group [mixed amphetamine salts versus placebo] by time period [phase 1 versus phase 2]) in a random regression model. The interaction term was not significant for the CGI improvement score (t=0.93, df=1, 55, p=0.34). Therefore, whether patients received mixed amphetamine salts in the first or second phase of the crossover trial was ignored, and the effect of mixed amphetamine salts was compared with that of placebo for all patients. For ADHD symptoms measured by the CGI improvement score, the improvement while patients took mixed amphetamine salts was 1.9 points greater than while they took placebo. This difference was significant (t=–9.2, df=28, p<0.0001) (
Table 2).
For Young Mania Rating Scale ratings, subjects randomly assigned to receive mixed amphetamine salts had a 3.4-point (SD=3.3) improvement in the Young Mania Rating Scale score during the first phase of the crossover trial, and those randomly assigned to receive placebo had a 1.5-point (SD=3.3) improvement, which was not significantly different from improvement with mixed amphetamine salts (t=–1.5, df=28, p=0.13). The treatment group-by-time interaction was not significant for the Young Mania Rating Scale score (F=0.01, df=1, 55, p=0.93), so the effect of mixed amphetamine salts was compared with that of placebo for all patients without regard for the order of administration. Patients did not improve significantly more while taking mixed amphetamine salts than while taking placebo (t=–1.4, df=29, p=0.17).
The few side effects reported during the course of the study largely occurred during the open-label and crossover trials. All side effects were transient (<1 month in duration) and reported as being low to moderate in severity and frequency. Reported side effects included abdominal pain (N=2), diarrhea (N=1), nausea (N=1), appetite increase (N=2), headache (N=1), drowsiness (N=2), difficulty falling asleep (N=1), irritability (N=1), and rash (N=1).
Follow-Up Phase
The open-label, 12-week follow-up phase was used to determine if coadministering mixed amphetamine salts with divalproex sodium would continue to be safe and effective. In this phase, 23 patients continued taking both divalproex sodium and open-label mixed amphetamine salts for 12 additional weeks. Dose adjustments for mixed amphetamine salts were allowed during the follow-up phase, and whole divalproex sodium oral doses were kept constant whenever clinically feasible. Our aim in maintaining steady divalproex sodium oral doses was to determine whether mixed amphetamine salts increased the metabolism of valproic acid.
Despite our desire to maintain constant oral doses, two patients had increases of 125 mg/day of divalproex sodium during the follow-up phase, and one had a decrease of 125 mg/day. The median dose of divalproex sodium was 750 mg/day at the end of the open-label divalproex sodium phase and at the end of the follow-up phase. When mixed amphetamine salts was added to divalproex sodium, there was no significant change in valproic acid serum levels. Among the 23 subjects taking both divalproex sodium and mixed amphetamine salts during the follow-up phase, the average valproic acid serum level was 82.9 μg/ml (SD=14.4) at the end of the 8-week open-label phase (i.e., no mixed amphetamine salts) and 82.4 μg/ml (SD=18.8) at the end of the follow-up (week 24) (i.e., mixed amphetamine salts present). The change in valproic acid serum level was not significant (t=–0.09, df=21, p=0.93).
Nearly one-half of the patients (45%) taking both divalproex sodium and mixed amphetamine salts required an increase in the dose of mixed amphetamine salts during the follow-up, although overall the doses used to successfully treat concurrent ADHD were less than expected. The average dose at the end of the study follow-up was 14.5 mg/day (SD=5.8). One patient experienced a manic exacerbation after 4 weeks of taking mixed amphetamine salts (highest Young Mania Rating Scale score=25; study week 12). For this patient, manic symptoms resolved within 4 weeks after mixed amphetamine salts was discontinued. The mean Young Mania Rating Scale score at week 16 (4 weeks after the crossover trial) was 5.6 (SD=2.9), at week 20 it was 4.8 (SD=3.9), and at week 24 (end of follow-up) it was 6.1 (SD=6.2).
Discussion
This study revealed that 8 weeks of open treatment with divalproex sodium monotherapy was associated with reductions in the symptoms of mania in pediatric patients with bipolar disorder and comorbid ADHD (p<0.0001) but with only minimal to no improvement in ADHD symptoms. This finding suggests that these patients have two distinct disorders, at least from the perspective of treatment response. Thirty-two (80%) of the 40 subjects treated with divalproex sodium alone had a ≥50% reduction in Young Mania Rating Scale baseline scores. This improvement rate is consistent with or higher than the previously published response rates (53%–80%) in open-label divalproex sodium treatment
(8–
10,
15). Medication doses were titrated to clinical response. Weekly valproic acid serum levels were monitored for safety but were not used to adjust oral doses if clinical response was acceptable.
During the 8 weeks of divalproex sodium monotherapy, ADHD symptoms were minimally improved. Only three of 40 patients achieved a response in ADHD symptoms with divalproex sodium alone (CGI improvement score for ADHD of 1 or 2 [very much improved or much improved]). Of these three responders, two still had significant ADHD symptoms as evidenced by scores on the hyperactivity index of the Conners’ Teachers and Parents Rating Scales. Clinically, we observed that divalproex sodium did not worsen patients’ attention. To the best of our knowledge, no previous reports have assessed the response of ADHD symptoms to divalproex sodium or other mood stabilizers.
Turning to the double-blind crossover trial, mixed amphetamine salts (5 mg b.i.d.) was more effective than placebo (p<0.0001) when added to ongoing divalproex sodium to treat concurrent ADHD symptoms. Mixed amphetamine salts was well tolerated. Divalproex sodium did not seem to interfere with the positive effect of mixed amphetamine salts on ADHD, given the rate of response to mixed amphetamine salts for ADHD (indicated by a CGI improvement score of 1 or 2) of 89.6%, which approximates response rates in the literature for ADHD without bipolar disorder. No clinically significant problems occurred when mixed amphetamine salts was initiated after stabilization of the symptoms of mania with divalproex sodium.
To our knowledge, this is the first double-blind, placebo-controlled trial of a stimulant for the treatment of concurrent ADHD in the context of pediatric bipolar disorder. Our findings are consistent with a retrospective chart review
(18) and a small prospective trial
(19), but unlike Biederman et al.
(18), who found that tricyclic antidepressants improved ADHD symptoms but frequently exacerbated mania, mixed amphetamine salts did not have this adverse effect in the context of ongoing effective divalproex sodium treatment for mania, except in the one of 23 patients in the 12-week, open-label follow-up who did experience a return of manic symptoms. This return of manic symptoms was the only adverse event that resulted in discontinuing mixed amphetamine salts.
This study had several limitations. Mixed amphetamine salts at a dose of 5 mg by mouth b.i.d. was likely not at a maximally effective dose (i.e., results might have been more robust with larger doses). In fact, in the follow-up, the dose of mixed amphetamine salts was raised further for 45% of patients. Even with the relatively low mixed amphetamine salts doses used in the crossover trial, patients demonstrated significant improvement in ADHD symptoms. Higher doses might have been associated with better symptom reduction and/or with more manic symptom breakthrough.
Second, divalproex sodium doses were not “pushed,” so that even more improvement in mania might have occurred at higher doses. However, in this study, a substantial proportion of the subjects experienced clinically significant improvement that was comparable to that found in other studies.
The generalizability of the findings is limited. The study included a small number of very symptomatic outpatients who presented to a single academic medical center. More severely ill patients requiring hospitalization were excluded. Furthermore, the results do not sufficiently address the long-term outcomes and safety of the combination of divalproex sodium and mixed amphetamine salts, although the 12-week follow-up data are encouraging. Whether either divalproex sodium or mixed amphetamine salts can be reduced in dose or entirely eliminated in the longer term deserves study.
These results indicate, first, that patients with pediatric bipolar disorder and concurrent ADHD can be safely and effectively treated with divalproex sodium to achieve control of their manic symptoms, although divalproex sodium monotherapy was not particularly effective for treatment of ADHD symptoms. Second, mixed amphetamine salts was effective and safe when added to divalproex sodium for the treatment of ADHD. Finally, the modest open-label, longer-term outcome data suggest continuing benefit for the combination of divalproex sodium and mixed amphetamine salts for both mania and ADHD. Additional information is needed on the optional dosing of both divalproex sodium and mixed amphetamine salts for these patients. A larger, more generalizable trial is indicated.