Neuropsychological deficits have been consistently identified at the onset of psychosis
(1–
4). Longitudinal studies of first-episode patients also suggest that these deficits do not change over time
(5,
6), implying that neuropsychological deficits are trait related and unlikely to be explained by the effects of medication. The neurodevelopmental notion of schizophrenia would predict that such deficits occur before the onset of illness. However, to date there have been only a few studies that, before the onset of illness, have examined cognitive measures in people at risk for the development of psychosis. These studies include the Edinburgh High Risk Study
(7), New York High-Risk Project
(8,
9), and Dunedin Multidisciplinary Health and Development Study
(10). In the reports by the Edinburgh group, slightly lower levels of global cognitive function were identified in a high-risk cohort than in a group of matched comparison subjects
(7). When this difference was controlled for, the high-risk group was significantly impaired only on a global memory test (Rivermead Behavioural Memory Test) and on a sentence completion test (Hayling Sentence Completion Test) that implicates working memory. However, none of the subjects in that study had become acutely psychotic at the time of publication; this highlights one of the problems of research in high-risk populations, namely, that it remains unclear to what extent neuropsychological deficits identified premorbidly are predictive of the later onset of schizophrenia.
The New York High-Risk Project
(8,
9) has explored this problem by following up a cohort of children into adulthood. The results demonstrated that childhood deficits in attention and short-term memory, measured at around 9 years of age, distinguished those who developed a schizophrenia-related psychosis more than 20 years later
(9). The Dunedin Multidisciplinary Health and Development Study has also identified lower intelligence and receptive language skills in children between the ages of 3 and 9 years who later fulfilled criteria for schizophreniform disorder at age 26
(10). While these studies provide evidence that early neuropsychological deficits are markers of impending illness later in life, it is unclear what the specificity of such deficits is for schizophrenia per se. Moreover, their predictive power at this phase of illness is likely to be weak.
These studies were limited by reliance on genetic vulnerability (in the case of the New York and Edinburgh studies) and the long follow-up period of 25–30 years, with high attrition rates and low rates of transition to psychosis (6%–15%). More recently, an alternative high-risk strategy has been adopted
(11). This “close-in” strategy identifies people putatively at very high risk of psychosis through a combination of trait and state risk factors. The advantage of this strategy is that it yields a much higher rate of transition to psychosis than family history alone and does so within a relatively short follow-up period, e.g., a 41% transition rate to psychosis within 12 months
(12). It has therefore been labeled the “ultra-high-risk strategy” to distinguish it from the traditional high-risk strategy, described later in this article and elsewhere
(12).
We have previously published reports of the impaired olfactory identification
(13) and poorer working memory
(14) of this ultra-high-risk population and preliminary results from a larger battery of cognitive tests
(15–
17). In the current study we examined neuropsychological ability in subjects at ultra-high risk of psychosis and in healthy comparison subjects, and we compared the ultra-high-risk individuals who became psychotic to those who did not develop a psychotic illness. Because olfactory identification has been shown to be poorer in individuals who not only develop psychosis but are diagnosed with schizophrenia
(13), we also examined performance by diagnostic outcome. Consistent with the neurodevelopmental hypothesis, we predicted that cognitive deficits would be most apparent in the individuals who subsequently developed first-episode psychosis.
Method
Subjects
Subjects were recruited between April 1995 and August 1998 and consisted of two groups comprising a total of 135 people, as follows: 1) 37 comparison subjects, previously described
(18), who were recruited from a local technological college, by advertising in local bulletins, and from ancillary staff and their families, and 2) 98 individuals at ultra-high risk for development of psychosis, consisting of consecutive referrals to the Personal Assessment and Crisis Evaluation clinic in Maribyrnong, Victoria, Australia
(19,
20).
The criteria for identification of the ultra-high-risk cohort and the rationale for these criteria have been previously described
(11); the participants met the criteria for at least one of three groups at intake, determined by “specific state” and/or trait risk factors for psychosis. The three groups were those with 1) trait plus state risk factors (i.e., genetic risk plus decrease in functioning), 2) attenuated symptoms, and 3) brief, limited intermittent psychotic symptoms. The criteria met by the subjects were as follows: trait plus state, 15.3%; attenuated symptoms, 44.9%; brief, limited intermittent psychotic symptoms, 13.3%; trait plus state and attenuated symptoms, 11.2%; trait plus state and brief, limited intermittent psychotic symptoms, 11.2%; attenuated symptoms and brief, limited intermittent psychotic symptoms, 1.0%; all three risk factors, 3.1%. All patients with ultra-high risk were between the ages of 14 and 29 years, had not experienced a previous psychotic episode (treated or untreated), and reported English as the preferred language. In order to identify the onset of acute levels of psychosis in the ultra-high-risk group, operationalized criteria for the onset of psychosis have been defined
(11,
19). The ultra-high-risk subjects were seen regularly by their clinic case managers, who monitored changes in mental state. These subjects underwent comprehensive psychopathology assessment at regular (1–6-month) intervals after the baseline assessment to detect the onset of acute psychotic symptoms. They were thus subsequently divided into two subgroups, depending on the outcome at the most recent follow-up interview (at least 12 months after baseline): ultra-high-risk psychotic, N=34 (34.7%), and ultra-high-risk nonpsychotic, N=64 (65.3%).
The predominant diagnoses in the ultra-high-risk psychotic group were schizophrenia and schizophreniform psychosis (N=18), and together these individuals made up a schizophrenia spectrum group. Ten of the ultra-high-risk patients with psychosis were diagnosed with affective psychosis (five with major depression with psychotic features, four with bipolar disorder with psychotic features, one with schizoaffective disorder), while another six were diagnosed with psychosis not otherwise specified (N=3), substance-induced psychosis (N=1), or brief psychotic disorder (N=2). These participants made up the “other psychosis” group. The majority of the ultra-high-risk patients without psychosis had no current diagnosis at follow-up (N=49), while the remaining subjects were diagnosed with major depressive disorder (N=4), generalized anxiety disorder (N=2), panic disorder (N=2), obsessive-compulsive disorder (N=1), social phobia (N=2), dysthymia (N=2), adjustment disorder (N=1), or posttraumatic stress disorder (N=1).
Subjects were excluded from the study if they had 1) documented neurological disorder, 2) a past history of head injury with loss of consciousness, 3) an estimated premorbid IQ below 70, 4) documented poor eyesight or hearing, or 5) for comparison subjects, a personal history of axis I psychiatric illness or documented family history of psychotic illness.
After complete description of the study to the subjects, written informed consent was obtained according to the Royal Park Psychiatric Hospital research and ethics committee guidelines.
Measures
Cognition
All subjects were assessed with the following measures by fully qualified neuropsychologists or clinical psychologists (W.J.B., S.M.F.):
1.
Premorbid IQ. The National Adult Reading Test
(21) provided an estimate of premorbid intellectual ability. Australian norms adjusted for educational level
(22) were used to calculate subject scores.
2.
Current IQ. A short form of the WAIS-R proposed by Ward
(23) (including the information, picture completion, block design, arithmetic, digit span, similarities, and digit symbol subtests) provided estimates of current verbal, performance, and full-scale IQs.
3.
Attention and executive functioning. The Stroop Color and Word Test
(24) provided a measure of response inhibition, an interference score calculated as the number of color-incongruent words divided by the number of color-congruent words (trial D/trial B) read in 45 seconds. The Controlled Oral Word Association Test
(25) provided a measure of verbal fluency, and Trail-Making Test parts A and B
(26) assessed visuomotor speed and task-switching ability.
4.
Learning and memory. Subtests (logical memory I, paired associates I, and visual reproduction I) from the Wechsler Memory Scale—Revised (WMS-R)
(27) provided measures of new verbal learning and of visual and verbal memory function. The Rey Auditory Verbal Learning Test
(28) modified to three trials was used to assess new verbal learning capacity and delayed recall.
Psychopathology
The Brief Psychiatric Rating Scale
(29) and the Scale for the Assessment of Negative Symptoms (SANS)
(30) were used to assess psychopathology in the ultra-high-risk subjects. All measures were administered to the subjects in this group within 3 weeks of acceptance to the program.
Statistical Analysis
Baseline demographic characteristics other than gender and premorbid IQ were compared in the ultra-high-risk subjects and the comparison subjects by using analysis of variance (ANOVA). For gender, the data were compared by using the chi-square test. Neuropsychological test scores were combined into cognitive domains and subjected to multivariate analyses of covariance (MANCOVAs) that controlled for age and/or premorbid IQ as necessary. Subsequent analyses of covariance (ANCOVAs) compared individual test scores, with post hoc comparisons performed by using Bonferroni tests. The exception was the WMS-R visual reproduction subtest, the results of which were not normally distributed. The data from this test were therefore analyzed by using the Kruskal-Wallis test and Mann-Whitney U for post hoc comparisons. Effect sizes are reported as Cohen’s d
(31).
Results
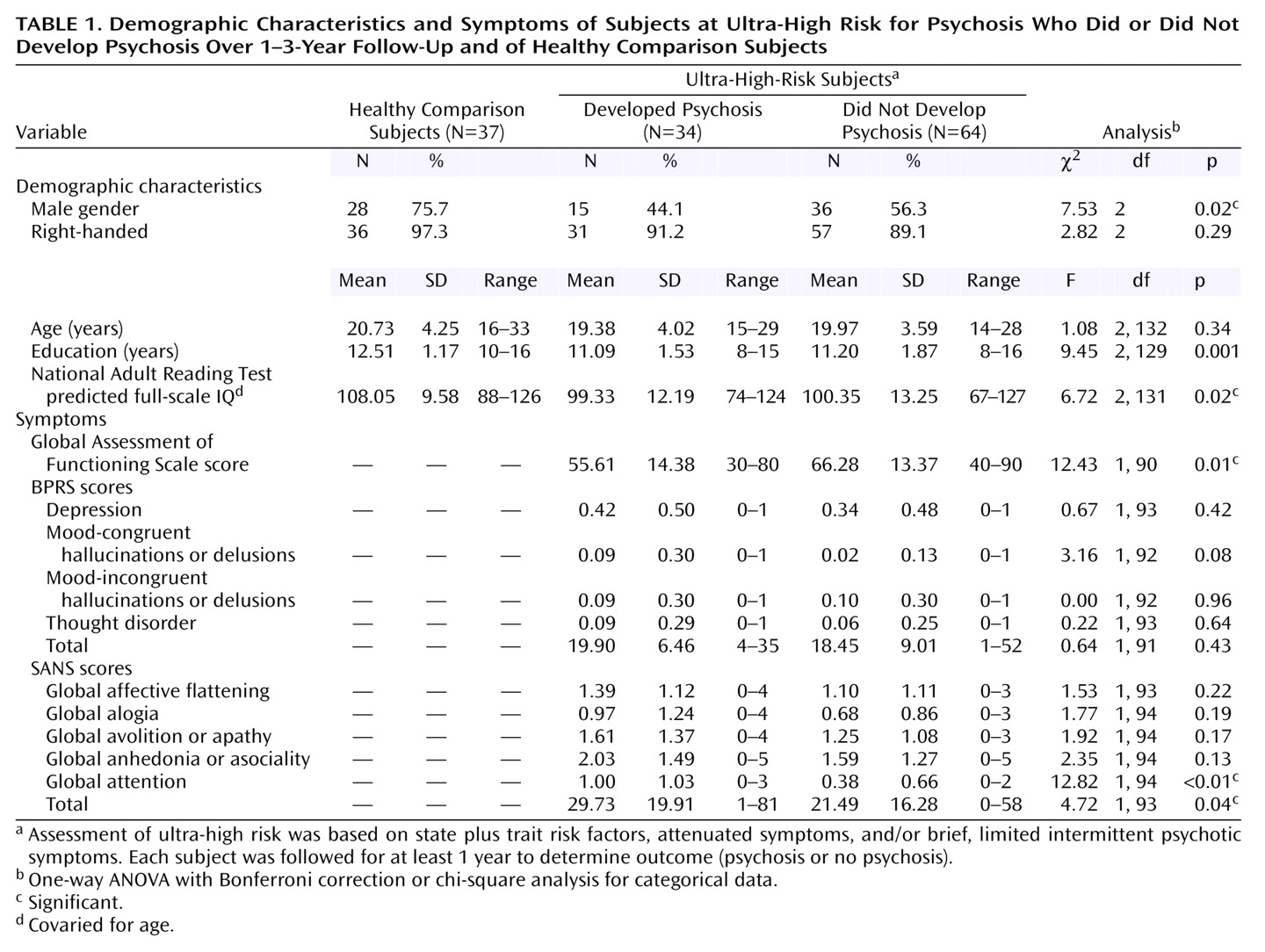
Table 1 lists the baseline demographic and clinical characteristics and premorbid IQ of the ultra-high-risk and comparison subjects. The two groups differed on gender (with lower proportions of males in the clinical groups), educational level, and premorbid IQ estimated by the National Adult Reading Test. Therefore, the estimated premorbid IQ was used as a covariate in further analyses (unless otherwise stated). In addition, the ultra-high-risk patients who developed psychosis had significantly higher total SANS scores and significantly lower Global Assessment of Functioning Scale (GAF) scores than the nonpsychotic ultra-high-risk group.
Global Cognitive Performance
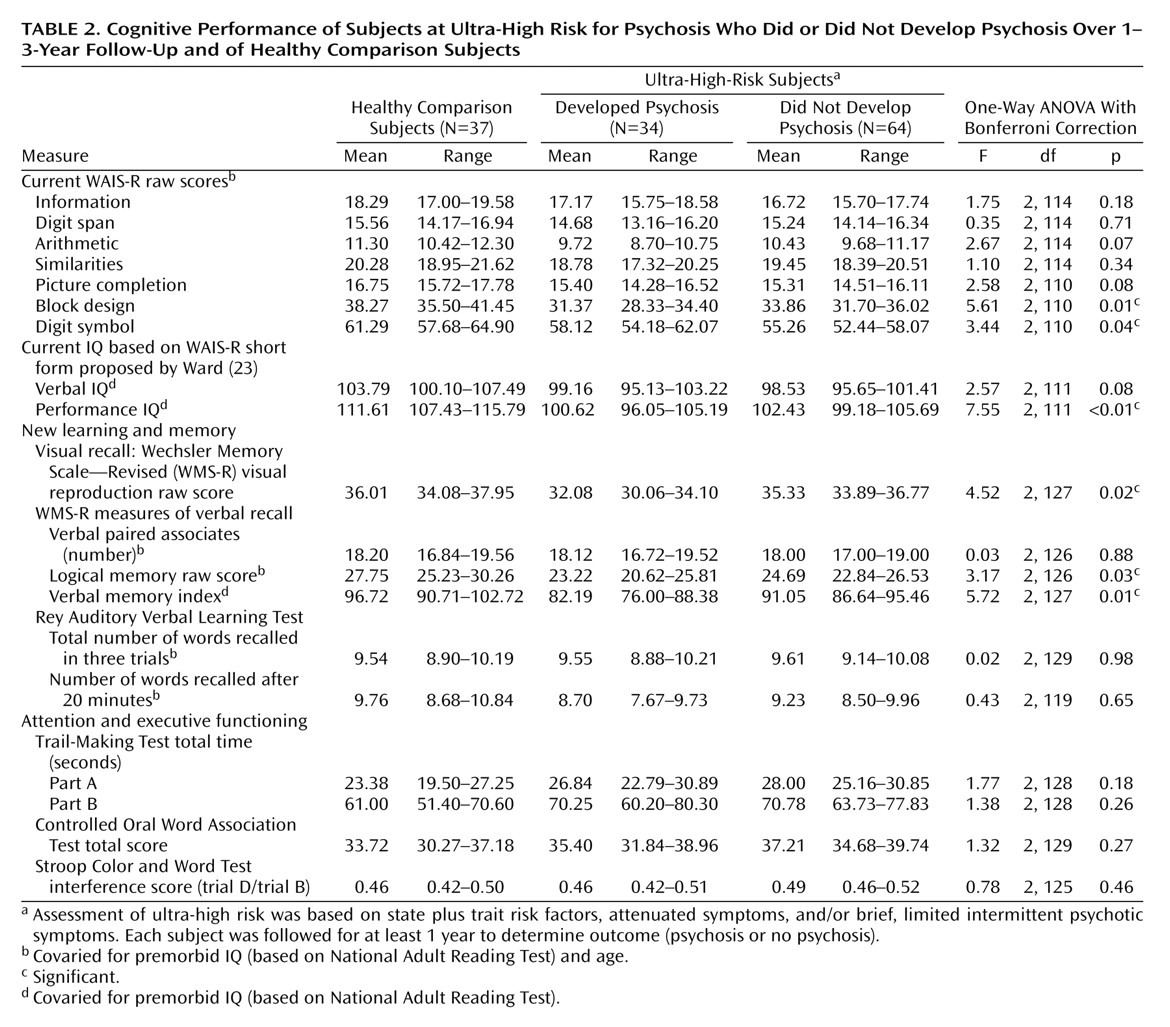
Baseline neuropsychological data for the three groups (ultra-high-risk psychotic, ultra-high-risk nonpsychotic, and comparison subjects) are shown in
Table 2. After premorbid IQ was controlled for, a MANCOVA examining verbal and performance IQs revealed a significant group effect (F=3.79, df=4, 220, Wilks’s lambda=0.88, p=0.005). A subsequent ANCOVA revealed significantly worse performance (visuospatial) IQ in both the psychotic and nonpsychotic ultra-high-risk groups than in the comparison subjects (F=7.55, df=2, 11, p<0.05, Bonferroni corrected). A deficit in verbal IQ fell short of significance.
A MANCOVA exploring the raw scores from the three subtests contributing to the performance IQ (with age and premorbid IQ controlled for) also revealed a significant group effect (F=2.97, df=6, 216, Wilks’s lambda=0.85, p=0.008). Subsequent ANCOVAs on the individual subtests demonstrated a significant group effect only for block design (F=5.61, df=2, 110, p<0.05) and digit symbol (F=3.44, df=2, 110, p<0.05), although the effect for picture completion approached significance. Specifically, block design was significantly worse in both the psychotic and nonpsychotic ultra-high-risk groups than in the comparison subjects, whereas for the digit symbol subtest it was the nonpsychotic patients with ultra-high risk who performed more poorly (p<0.05, planned post hoc pairwise comparisons, Bonferroni corrected). Investigation of the effect sizes, in relation to the comparison subjects, for the digit symbol subtest confirmed this analysis (psychotic ultra-high-risk group, d=–0.29; nonpsychotic ultra-high-risk group, d=–0.57).
A MANCOVA exploring the raw scores from the four subtests contributing to the verbal IQ (with age and premorbid IQ controlled for) did not reveal a significant group effect (F=1.08, df=8, 222, Wilks’s lambda=0.93, p=0.38).
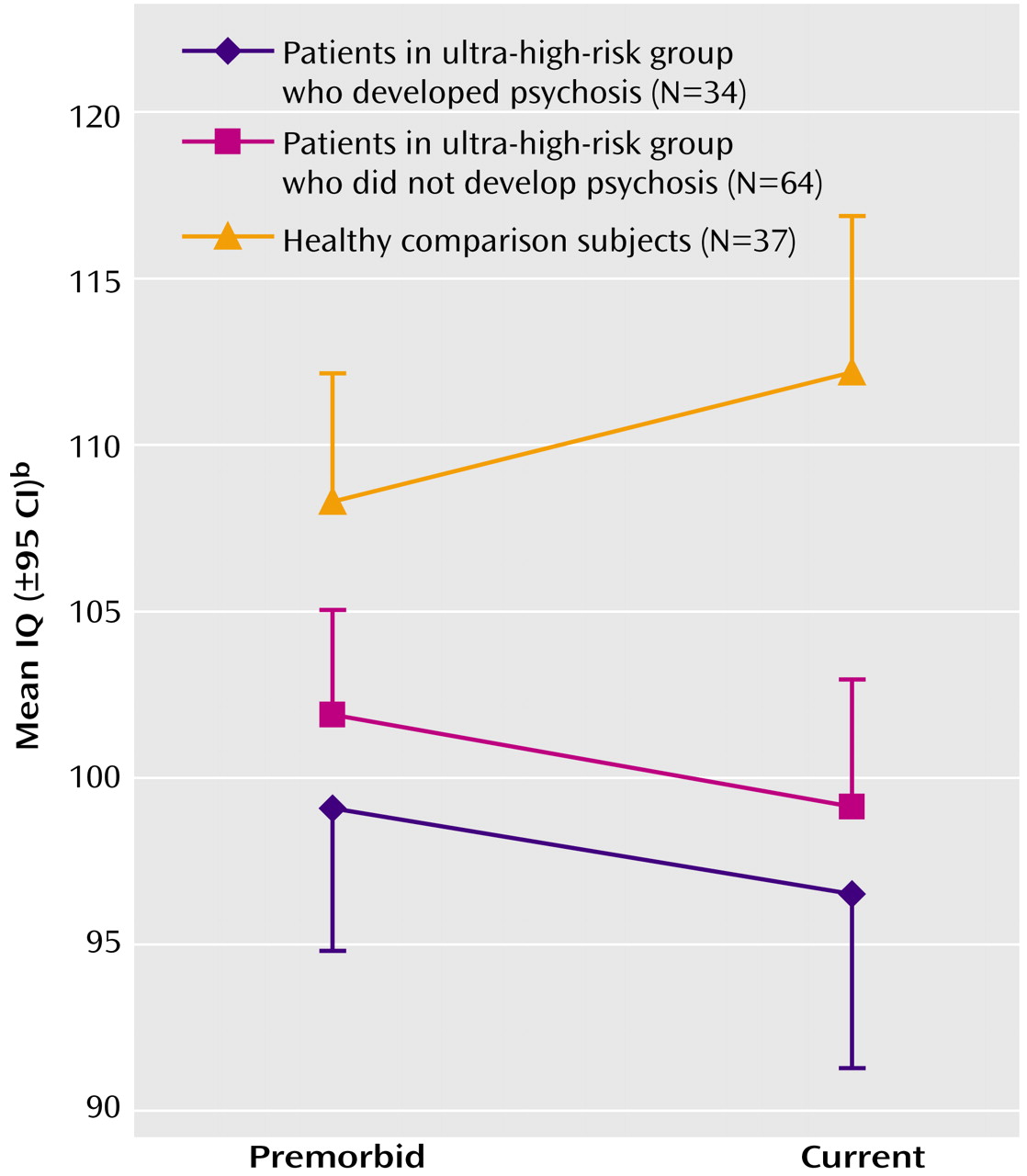
In order to explore a possible decline in global function from premorbid levels, a repeated-measures ANCOVA (controlling for age) of the estimated premorbid IQ (National Adult Reading Test) and current full-scale IQ (WAIS-R) was performed. This demonstrated a significant effect of time (with Greenhouse-Geisser adjustment, F=8.58, df=1, 115, p=0.004) and of group (F=10.58, df=2, 115, p<0.001). In addition, there was a significant group-by-time interaction effect (Greenhouse-Geisser, F=5.05, df=2, 115, p=0.008), which was explained by the fact that both ultra-high-risk groups did not show the improvement in IQ between the premorbid and current measurements that was demonstrated by the comparison group (
Figure 1).
Memory
An initial ANCOVA examining the verbal memory index from the WMS-R revealed a significant group effect (F=5.52, df=2, 127, p=0.05) (
Table 2), whereby the ultra-high-risk subjects who developed psychosis scored significantly lower than the comparison subjects. In addition, the psychotic patients scored nonsignificantly worse than the nonpsychotic patients in the ultra-high-risk group (p=0.07, Bonferroni corrected).
Exploration of the two subtests that make up the verbal memory index (logical memory and verbal paired associates) using a MANCOVA that controlled for age and premorbid IQ did not demonstrate a significant group effect (F=1.97, df=4, 250, Wilks’s lambda=0.94, p=0.10). However, inspection of the effect sizes in relation to the comparison subjects for these two subtests suggested that the impairment in the verbal memory index among the ultra-high-risk patients was solely due to poorer logical memory scores (logical memory effect sizes: psychotic patients, d=–0.60; nonpsychotic patients, d=–0.41), particularly in the psychotic group, while performance on verbal paired associates was completely normal (verbal paired associates effect sizes: psychotic patients, d=–0.02; nonpsychotic patients, d=–0.05). This result remained when we examined hard (unrelated) word pairs alone (F=0.16, df=2, 130, p=0.86).
Because the raw scores on the visual reproduction subtest were not normally distributed, a comparison of group means using ANCOVA was inappropriate. Instead, the nonparametric Kruskal-Wallis test was used, which indicated a significant group effect (χ2=20.0, df=2, p<0.001). Post hoc Mann-Whitney comparisons of each pair of groups demonstrated that the comparison group outperformed both the psychotic (U=273.5, p<0.001) and nonpsychotic (U=686, p=0.001) patients in the ultra-high-risk group. In addition, the psychotic patients performed significantly worse on this task than the nonpsychotic patients in the ultra-high-risk group (U=728.5, p=0.03).
The Rey Auditory Verbal Learning Test was analyzed with a repeated-measures ANCOVA covarying for age and premorbid IQ. While this revealed a nearly significant effect of trial (Greenhouse-Geisser, F=2.68, df=1.95, 251.02, p=0.07), there was no group effect and no group-by-trial interaction (for both, p>0.85). An additional repeated-measures ANCOVA that used the last learning trial and the delayed recall trial also failed to find significant effects (for all, p>0.15).
Attention and Executive Functioning
Speed of processing and switching ability, as measured by the trails A total time, trails B total time, and their ratio (trails B time divided by trails A time), were examined by using a MANCOVA that covaried for age and premorbid IQ. This failed to identify a significant group effect (F=0.73, df=6, 252, Wilks’s lambda=0.97, p=0.63). Similarly, no group effect was identified for performance on a test of executive functioning (the Controlled Oral Word Association Test) or a test of attention and response inhibition (Stroop Color and Word Test interference), as assessed by individual ANCOVAs (for both, p>0.25).
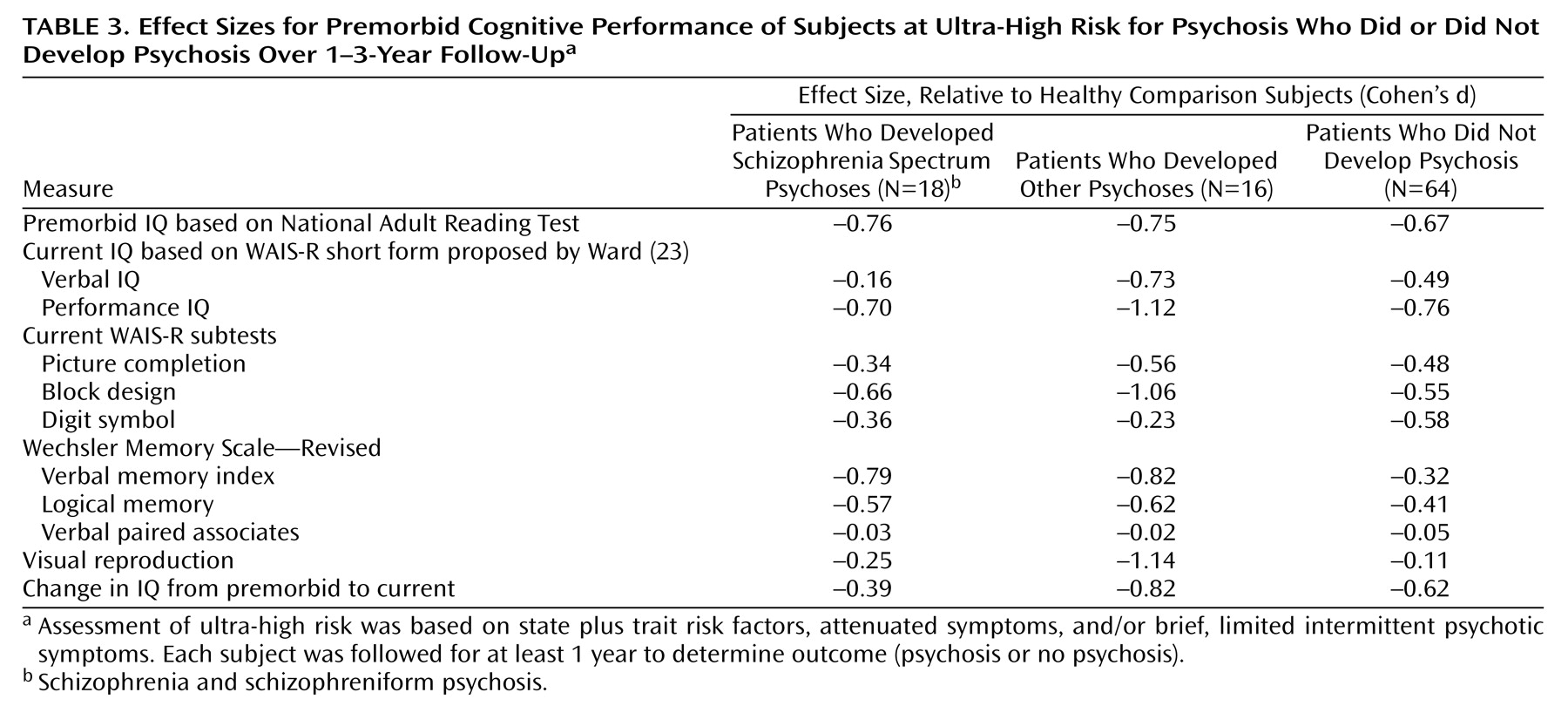
Subanalysis by Outcome Diagnosis
The effect sizes (Cohen’s d) for comparisons of the three subgroups within the ultra-high-risk patients (those with schizophrenia spectrum disorders, those with other psychoses, and those who were nonpsychotic) with the healthy subjects are shown in
Table 3. For most tests the effect sizes for the two psychotic groups were broadly comparable. The exceptions were verbal IQ and the WMS-R visual reproduction subtest, on which the patients who developed non-schizophrenia-related psychoses were markedly more impaired.
Subanalysis by Gender
Because there was a significantly higher proportion of males in the comparison group than in either ultra-high-risk group, we repeated all the preceding analyses while using sex as another between-groups factor. This revealed only a sex-by-diagnosis interaction for the MANCOVA of the verbal and performance IQs. Further inspection of the data revealed a single female outlier with a large discrepancy between her verbal and performance IQs. Removing this outlier also removed the significant sex-by-diagnosis interaction. Reanalysis of the significant findings without this person did not change the results.
Discussion
To our knowledge, this is the first published large-scale study to examine a broad range of neuropsychological functioning in individuals at ultra-high risk of developing psychosis. We have shown that, in relation to a comparison group, the ultra-high-risk group had significant impairments in premorbid functioning, performance IQ, and aspects of visual and verbal new learning. In particular, problems in verbal learning were specific to the individuals who developed psychosis over the follow-up interval. Our data are broadly consistent with those from previous work examining cognitive deficits in prepsychotic individuals
(7–
10,
32), although the exact domains of impaired functioning differ across research centers. These data are also consistent with our own findings on olfactory functioning
(13), working memory
(14), and attention
(15–
17). However, it remains to be shown that this cluster of vulnerabilities is specific to the psychotic spectrum of emerging psychopathology.
The ultra-high-risk patients who became psychotic performed significantly more poorly than those who did not develop psychosis on two measures of immediate memory—the WMS-R verbal memory index (derived from the logical memory and verbal paired associates subtests) and the visual reproduction subtest. Subanalyses of the two tests making up the verbal memory index indicated that the difference in performance was completely explained by impairments in logical memory, since both clinical groups scored at the level of the comparison subjects on the verbal paired associates subtest. The logical memory and visual reproduction subtests are similar in that both require the rapid processing of incoming information and its efficient organization for accurate recall. In addition, both are unstructured in the manner in which recall is initiated (in contrast to the verbal paired associates subtest, which incorporates built-in cueing) and require single trial learning (again in contrast to the verbal paired associates and the Rey Auditory Verbal Learning Test). Moreover, these tasks may reflect the contribution of meaning to efficient retention and recall. Taken together, these findings suggest that the application of efficient organizational strategies is compromised in the ultra-high-risk individuals who become psychotic, implicating vulnerabilities in prefrontal networks. This suggestion is consistent with our findings of impaired spatial working memory
(14), poorer olfactory identification
(13), and lower frontal gray matter volumes
(33) in this population.
The finding of unimpaired performance on the verbal paired associates subtest in both clinical groups (even on hard pairs alone) is particularly interesting given the focus on hippocampal structure and functioning in psychotic disorders. Learning of paired associates is considered a probe of hippocampal functioning
(34), and therefore these data suggest functional integrity of this region before the onset of psychosis. This further suggests that there may be a decline in this ability associated with development of the disorder, which is consistent with our previously published data demonstrating reduction in left medial temporal gray matter over the same period
(33). However, this hypothesis remains to be confirmed by longitudinal studies.
One of the most striking results from this study is the general lack of clinically significant cognitive impairments in the ultra-high-risk group. For example, their mean IQs were at the population mean, suggesting that the global cognitive deficits found after the onset of psychosis may not be key features of premorbid vulnerability. This finding contrasts with the results of the limited number of genetic and population-based high-risk studies
(7–
10) and needs to be replicated in other prodromal populations. However, while the analysis of the difference between estimated premorbid IQ and current full-scale IQ revealed an overall “increase” for the comparison subjects, there was a “decline” for both ultra-high-risk groups. In essence, this suggests that the National Adult Reading Test underestimated the IQ of the comparison group but, if anything, overestimated the IQs of the ultra-high-risk groups. This finding could potentially be attributed to a recent global decline in functioning, although the National Adult Reading Test may not be a good instrument for estimating premorbid ability in this age group because of the strong relationship between this measure and years of education.
The exploration of effect size differences between the subgroup who later developed schizophrenia and those who later developed other psychotic disorders does not suggest any diagnostic specificity—indeed, for most tasks the poorest performance was exhibited by the “other psychosis” subgroup. This is in contrast with our work on olfactory identification
(18), in which the poorest performance was seen in the subgroup of patients who later developed schizophrenia. However, the data from the current study are consistent with research in first-episode psychosis
(35–
37), which does not demonstrate diagnostic specificity in cognitive functioning.
These findings should be considered in the context of a number of limitations. First, one of the criteria for the selection of ultra-high-risk subjects was a recent reduction in global functioning (as measured by the GAF). This would be likely to bias our study toward finding impairments in the ultra-high-risk groups, although not necessarily a difference between the psychotic and nonpsychotic groups. There was also a significant difference between the gender distributions of the comparison group and the two ultra-high-risk groups. While subanalyses revealed no effect of gender, previous results suggest that visuospatial abilities may be more efficient in males
(38,
39), and therefore the differences in performance IQ and visual reproduction need to be examined further. Third, our results may have been confounded by the fact that 11 of the nonpsychotic subjects in the ultra-high-risk group received some treatment after baseline (low-dose antipsychotic and cognitive therapy), which may have affected their transition to psychosis
(40), i.e., some of these patients might have developed the illness if we had not intervened. However, it could be argued that this was the case for everyone in the study, as they all received some level of clinical care, and even supportive monitoring may affect the probability of transition to psychosis. Finally, use of standardized neuropsychological measures is less useful for the purpose of challenging more specific components of cognition.
In summary, we have demonstrated that significant impairments in cognitive functioning are apparent before the onset of psychotic illness and that they implicate the functional integrity of prefrontal regions. Future studies, using experimental tasks, need to address the components of the more generalized aspects of cognitive processing tested in this study. They will also need to explore the predictive capacity of these findings for the development of psychosis.