Borderline personality disorder is a severe psychiatric illness that affects approximately 2% of the general population. Studies with typical antipsychotic medications have revealed significant improvements in symptoms such as suspiciousness or impulsiveness. However, tolerability of these drugs is poor, which leads to high dropout rates
(1). Atypical antipsychotics have a more tolerant profile and foster greater long-term treatment compliance. In two double-blind, placebo-controlled clinical trials in patients with borderline personality disorder, olanzapine resulted in significant improvements. Nonetheless, 43%–68% of the subjects did not complete the studies
(2,
3).
Psychotherapeutic strategies are fundamental for treating personality disorders. Dialectical behavior therapy has proven its efficacy in borderline personality disorder treatment in several controlled studies
(1,
4). New strategies combining pharmacotherapy and psychotherapeutic interventions may help to reduce dropout rates and control the influence of psychotherapy. We carried out a single-center, randomized, double-blind, placebo-controlled study to compare the efficacy and safety of dialectical behavior therapy plus olanzapine or placebo in patients with borderline personality disorder.
Method
Of the 125 subjects referred from clinical services, 65 met inclusion criteria. Five of the 65 dropped out during the selection phase. Inclusion criteria were 1) meeting DSM-IV diagnostic criteria for borderline personality disorder as assessed by the Structured Clinical Interview for DSM-IV Axis II Disorders and the Revised Diagnostic Interview for Borderlines
(5); 2) age of 18–45 years; 3) without comorbid, unstable axis I disorder; 4) Clinical Global Impression (CGI) severity of illness
(6) score ≥4; 5) not receiving psychotherapy; 6) for female subjects, using medically accepted contraception.
Patients could continue treatment with benzodiazepines, antidepressants, and mood stabilizers, but doses could not be modified. Complete physical examination, laboratory tests, and pregnancy tests were performed, and written informed consent was obtained from all participating subjects.
This controlled clinical trial consisted of a selection phase lasting 4 weeks (baseline) and an experimental phase lasting 12 weeks. During the selection phase subjects had three evaluation visits to establish a preintervention baseline but underwent no therapeutic intervention. They were then randomly assigned to receive dialectical behavior therapy plus either olanzapine or placebo on a 1:1 ratio. Treatment doses were flexible and ranged from 5 to 20 mg/day.
Participants were evaluated every 2 weeks by an experienced psychiatrist and participated in weekly 150-minute group psychotherapy sessions led by two trained psychotherapists. The dialectical behavior therapy format was adapted from the standard version; two of the four types of intervention were applied: skills training and phone calls.
Assessments included several clinical scales such as the Hamilton Depression Rating Scale
(7) for affective symptoms, the Hamilton Anxiety Rating Scale
(8) for anxiety symptoms, and the CGI severity of illness scale
(6) to evaluate overall improvement. Data were also obtained on pragmatic variables regarding the subject’s most dysfunctional behaviors from biweekly behavioral reports: episodes of impulsivity/aggressive behavior, self-injuring behavior/suicide attempts, and visits to psychiatric emergency services. Safety was evaluated by assessing treatment-emergent adverse events and scales assessing extrapyramidal side effects.
All analyses were conducted on an intent-to-treat basis. The endpoint was based on a last-observation-carried-forward strategy. Patients were included in the analyses only if they had a baseline measure and at least one postbaseline measure. The chi-square test, Student’s t test, and analysis of variance (ANOVA) and covariance (ANCOVA) models were used to compare outcomes. All tests of hypotheses were performed with a two-sided significance level of 0.05.
Results
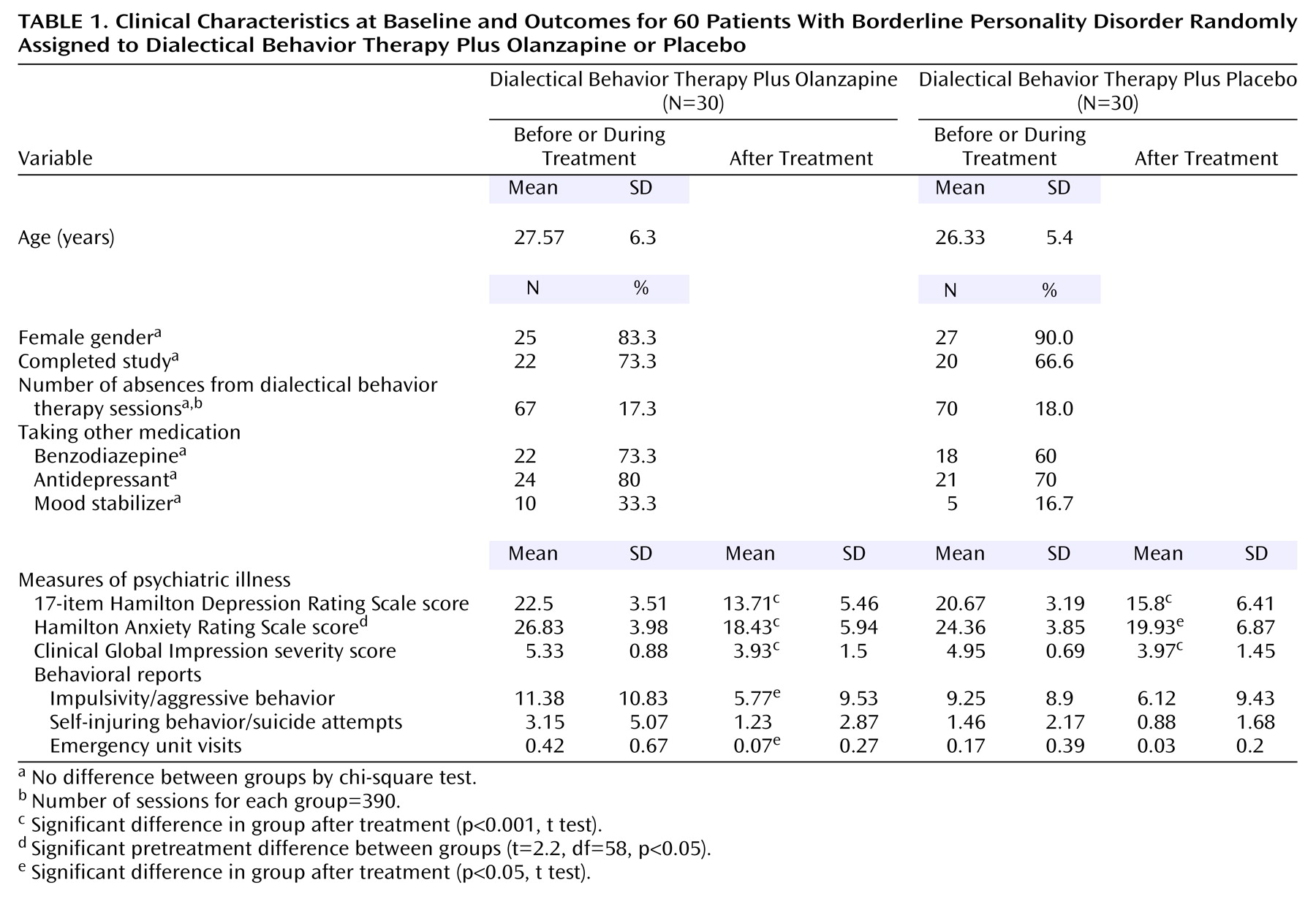
Sixty subjects were randomly assigned to dialectical behavior therapy plus olanzapine or placebo and started the experimental phase; 42 subjects (70%) completed the study. There were no between-group differences regarding demographic variables or concomitant treatments at baseline. Neither dialectical behavior therapy intervention time nor dropout rates differed significantly between the two groups (eight of the 30 patients who received olanzapine versus 10 of the 30 who received placebo dropped out before the end of the study) (
Table 1).
Table 1 summarizes the mean measures before and after intervention. There were no significant pretreatment differences between groups with the exception of Hamilton anxiety scale scores. With the intervention, both groups showed a significant improvement in most psychopathology scales. According to ANOVA of the differences between groups, the olanzapine-treated group showed a greater decrease in depressive symptoms according to Hamilton depression scale scores (F=4.24, df=3.44, 192.64, p=0.004). Given the between-group differences in pretreatment Hamilton anxiety scale scores, ANCOVA was performed by entering the baseline anxiety scores as covariants. A significant decrease in clinical anxiety in the olanzapine-treated group according to Hamilton anxiety scale scores was observed (F=3.57, df=3.39, 186.83, p<0.02).
In the behavioral reports, the olanzapine plus dialectical behavior therapy group experienced a significantly greater decrease in the frequency of impulsivity/aggressive behavior than the placebo plus dialectical behavior therapy group (F=2.82, df=3.68, 184.23, p=0.03). Self-injuring behavior/suicide attempts was decreased nonsignificantly in olanzapine-treated participants (F=2.42, df=2.49, 124.95, p=0.08).
The mean dose of olanzapine was 8.83 mg/day (SD=3.8, range=5–20). No differences were detected between groups with respect to secondary effects spontaneously reported by the subjects or in movement disorders. Olanzapine-treated patients experienced more weight gain than placebo-treated patients: 2.74 kg (SD=3.2, range=–9 to 7) versus –0.05 kg (SD=2.39, range=–8 to 3) (F=3.24, df=1.84, 103.55, p<0.05). Participants treated with olanzapine experienced a significantly greater increase in cholesterol levels: 0.28 mg/dl (SD=0.53, range=–1.03 to 1.34) versus –0.1 mg/dl (SD=0.65; range=–1.16 to 1.47) (F=3.38, df=1.94, 93.39, p<0.04).
Discussion
A combined psychotherapeutic/pharmacological approach for patients with borderline personality disorder appears to lower dropout rates and constitutes an effective treatment. Dropout rates in clinical trials using antipsychotics in patients with borderline personality disorder vary between 50% and 88%
(1). Including dialectical behavior therapy enhances the therapeutic relationship and has enabled us to improve patients’ treatment compliance, lowering the dropout rate to 30%. Furthermore, since clinical borderline personality disorder studies do not usually assess psychotherapy treatment, an active variable may become an uncontrolled variable.
Olanzapine was significantly superior to placebo in improving mood and anxiety symptoms and in reducing impulsivity/aggressive behavior. The reduction in clinical anxiety and impulsivity/aggressive behavior found is consistent with the findings of previous borderline personality disorder studies with olanzapine
(2,
3). Our results indicate that olanzapine also improves depressive symptoms in borderline personality disorder. Several studies have shown that olanzapine has mood-elevating properties in schizophrenia and depression, but in borderline personality disorder this antidepressant effect was not found in any controlled trials and in only one uncontrolled trial
(1).
The olanzapine dose used (8.83 mg/day) was well tolerated, and no increases in extrapyramidal symptoms were seen. Olanzapine-treated patients experienced a significant weight gain, but there was no dose-dependent relation. Increased levels of cholesterol were above normal reference intervals in only three patients. According to our data, patients with borderline personality disorder may benefit from higher doses of olanzapine without increasing secondary effects.
This study was designed to minimize the limitations typically found in this complex patient group. To achieve a representative group of borderline personality disorder patients we included only those from psychiatric services and emergency psychiatric units with moderate-to-high clinical severity and without unstable comorbid axis I disorders. Concomitant treatment with other drugs at stable doses was allowed, as was use of toxic substances without dependence criteria.
Because the rapidly changing symptoms and the episodic nature of borderline personality disorder make it difficult to establish the patient’s baseline status, several pretreatment assessments were made. Because there are no specific instruments to assess changes in borderline personality disorder, representative pragmatic variables were used as behavioral reports.
Study limitations are that the results cannot be fully extrapolated to inpatients, to patients with active comorbid axis I disorders, or to those with less clinically severe disorders. We are unaware of any possible drug-drug interactions, the “masking” effect of psychotherapy, or whether olanzapine is useful as maintenance treatment. Additional studies are needed to replicate these findings.