Hypofunction of the
N-methyl-
d-aspartate (NMDA)-type of glutamate receptors may contribute to pathophysiology in schizophrenia. Clinical studies with the NMDA/glycine-site agonists glycine and
d-serine
(1) indicate significant improvements in negative and cognitive symptoms of schizophrenia, supporting the concept that reduced activation of the glycine binding site of the NMDA receptor contributes substantially to ongoing symptoms. Since glycine is not actively transported across the blood-brain barrier, plasma levels of this compound are in equilibrium with brain levels
(2). Moreover, in several glycine trials in schizophrenia (reviewed in reference
1), low pretreatment plasma glycine levels predicted treatment response, suggesting that reduced glycine concentrations may be of particular importance.
Two recent studies
(3,
4) provide direct support for this contention. Sumiyoshi et al.
(3) demonstrated low glycine levels but normal total serine levels in medication-free patients with schizophrenia, leading to significantly lower glycine-serine ratios. Low serum glycine levels predicted higher levels of negative symptoms, reflected by Brief Psychiatric Rating Scale (BPRS) withdrawal-retardation scores. Hashimoto et al.
(4) demonstrated similar findings with
d-serine, which, like glycine, serves as an allosteric modulator of the NMDA receptor.
In addition to being associated with lower levels of positive NMDA modulators, schizophrenia may also be associated with higher levels of endogenous NMDA antagonists. In particular, elevated levels of homocysteine, which acts in part as an NMDA-glycine site antagonist
(5), may also contribute to symptom development. High homocysteine plasma levels are an established risk factor for cardiovascular, cerebrovascular, and Alzheimer’s disease and have been observed in schizophrenia
(6). Administration of folic acid, pyridoxine, and B
12, which help reduce homocysteine levels, may also have beneficial effects
(7).
To our knowledge, this is the first study to evaluate glycine and homocysteine levels and their correlations with symptom profile and medication status in a group of patients with schizophrenia. Serine levels, along with those of the related amino acid alanine, were obtained as comparison compounds and to allow assessment of glycine-serine ratios. Since patients receiving clozapine are reported to show less robust response to treatment with NMDA modulators than patients treated with other antipsychotics
(1), the present study was performed with patients receiving different medications at the time of recruitment to allow comparisons across medication status. Although
d-serine may serve as an additional amino acid of interest, it was not assessed separately in the present study because of technical limitations.
Method
The patient group consisted of 94 inpatients and outpatients with chronic schizophrenia diagnosed according to DSM-IV who did not have a history of neurological or cardiovascular disorders or substance abuse. All patients were being treated with antipsychotic drugs, including conventional neuroleptics (N=33), clozapine (N=9), and risperidone/olanzapine (N=52). The Positive and Negative Syndrome Scale
(8) was administered at the time of blood sampling. Comparison subjects consisted of 34 age- and gender-matched hospital and university employees. The study was approved by our institutional review board, and written informed consent was obtained from all subjects after complete description of the study.
To minimize the effects of diet, fasting morning blood samples were obtained from all study participants. Glycine, serine, and alanine plasma levels were measured by ion exchange chromatography with a Pharmacia Biochrom 20 amino acid analyzer (Biochrom, Cambridge, U.K.). Plasma homocysteine levels were measured by a solid-phase, competitive chemiluminescent enzyme immunoassay using the Immulite 2000 homocysteine kit on an Immulite 2000 analyzer (DPC Biermann, Friedberg, Germany).
Demographic comparisons were made with t tests or z-transformed Mann-Whitney U tests, as appropriate. Between-group and between-medication differences in amino acid levels were analyzed with analysis of variance; the between-subject factors were diagnostic group and gender, and age was the covariate, as indicated. The Tukey honestly significant test was used to assess post hoc medication effects.
Results
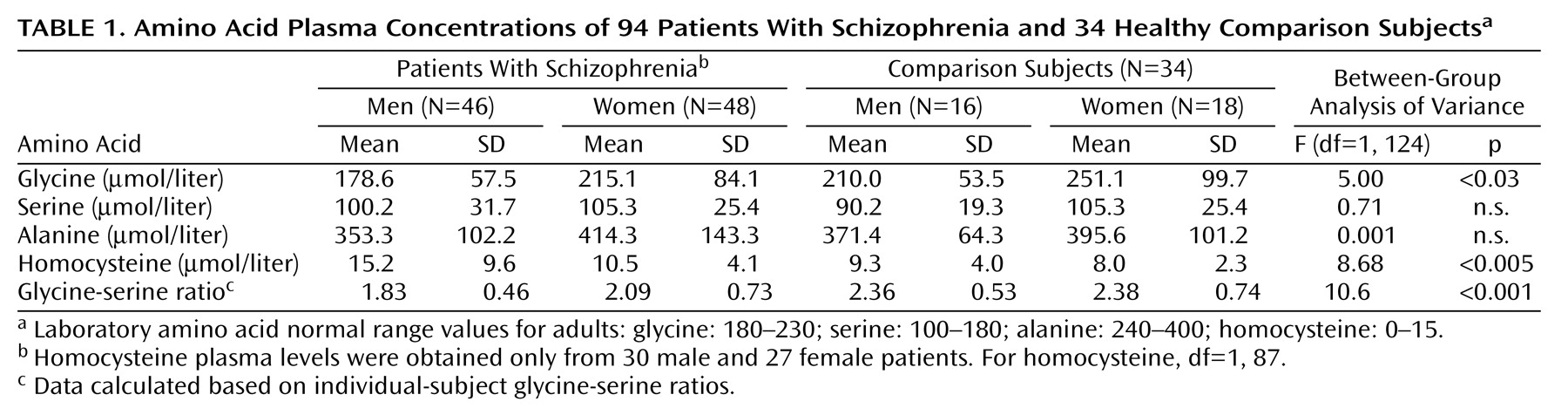
Mean ages were 42.7 years (SD=11.3) for patients and 42.7 (SD=1.9) for comparison subjects (t=0.0, p>0.9). Further, 48 (51.1%) of the 94 patients were women, compared with 18 (52.9%) of the 34 comparison subjects (z=0.2, p=0.8), reflecting successful employment of the matching procedure. Mean Positive and Negative Syndrome Scale positive, negative, and total symptom scores of patients were 23.4 (SD=73.4), 30.1 (SD=7.4), and 105.3 (SD=21.5), respectively.
Patients had significantly lower plasma levels of glycine, but not serine or alanine (
Table 1). Although overall glycine levels were different between men and women (F=7.43, df=1, 124, p<0.01), male and female patients had similarly lower glycine levels (15.0% and 14.3%, respectively) than male and female comparison subjects, leading to a nonsignificant gender-by-cohort effect (F=0.01, n.s.). The difference in glycine-serine ratios between patients (mean=1.97, SD=0.62) and comparison subjects (mean=2.37, SD=0.64) was also highly robust (F=10.6, df=1, 124, p=0.001) with no significant age (F=0.7, n.s.) or gender (F=0.9, n.s.) effects or gender-by-group interactions (F=0.9, n.s.). Low glycine levels correlated significantly with high levels of negative symptoms (r
s=–0.24, N=72, p=0.04), but not with severity of other symptom clusters.
As opposed to glycine levels, which were low in patients, homocysteine levels were high (
Table 1). Levels were higher overall for men than for women (F=4.9, df=1, 87, p=0.03). Further, homocysteine levels were 65% higher in male patients than male comparison subjects but only 25% higher in female patients than female comparison subjects, although the group-by-gender interaction was not significant (F=1.57, p=0.2). There were no significant correlations between homocysteine levels and either glycine levels or clinical symptoms. When entered into a logistic regression relative to diagnosis, the combination of glycine-serine ratio, homocysteine levels, and alanine levels led to an 82.2% rate of correct classification.
Antipsychotic medication type had no significant effect on absolute levels of any of the amino acids measured (all p>0.2). However, there was a significant medication effect on glycine-serine ratios even following covariation for age and sex (F=8.55, df=2, 84, p<0.001): ratio levels in the presence of clozapine were significantly higher than those in the presence of either conventional antipsychotics (p=0.001, Tukey) or other atypical antipsychotics (p=0.002, Tukey). Further, whereas ratio levels of patients receiving conventional (p=0.004, Tukey) or newer atypical (p=0.002, Tukey) antipsychotics were significantly lower values than comparison subjects, patients receiving clozapine had higher glycine-serine ratios.
Discussion
Our findings lead us to the conclusion that amino acid disturbances may contribute significantly to the pathophysiology of schizophrenia. This study supports previous findings
(3,
6) and indicates that low glycine and high homocysteine levels may coexist in patients with schizophrenia. Further, this study demonstrates that low glycine levels persist even in medicated patients with schizophrenia, although levels may differ by medication types. In particular, glycine levels may be higher among patients receiving clozapine, potentially explaining the differential effectiveness of NMDA modulators when used in combination with clozapine or with other antipsychotics.
The low glycine and glycine-serine ratios observed in the patient group are similar to those reported by Sumiyoshi et al.
(3) among unmedicated patients. Furthermore, the correlation found between low glycine levels and high Positive and Negative Syndrome Scale negative symptom scores corroborates the negative correlation between glycine levels and BPRS withdrawal-retardation scores reported by these authors. It is thus unlikely that the low glycine and glycine-serine ratios found in the present study were the result of ongoing medication. The finding of normal glycine-serine ratios in clozapine-treated patients is all the more intriguing given the low levels observed in both unmedicated patients and those receiving other typical or atypical antipsychotics. Since low glycine levels predict, in general, greater clinical response to glycine-like agents, this finding may explain the less robust effect of glycine-site agonists when added to clozapine in the treatment of schizophrenia. Clozapine has been found to inhibit glycine transport at system A-like transporters
(9), providing a potential mechanism for the present results.
Homocysteine has multiple dose-dependent effects on brain function, including interaction with NMDA receptors. At high concentrations, homocysteine may activate the NMDA receptor glutamate site, leading to increased susceptibility to excitotoxicity
(5). However, at lower concentrations homocysteine also has the ability to act as a competitive antagonist at the NMDA receptor coagonist glycine site
(5,
10). Neurodevelopment studies
(10) indicate that adverse homocysteine effects are significantly blocked by glycine, reflecting reversal of the inhibitory actions of homocysteine. Our findings indicate that homocysteine plasma levels are high in schizophrenia and that this may occur independently from coexisting low glycine levels.
The present study is limited by cross-sectional design and small overall number of subjects. Nevertheless, these preliminary findings warrant larger-scale investigation and suggest that combined treatment strategies aiming at counteracting detrimental effects of low glycine (e.g., high-dose glycine treatment) and high homocysteine (e.g., folic acid supplementation) levels may be explored in schizophrenia.