From computerized tomography (CT) studies done in the 1980s through the mid-1990s, cross-sectional and longitudinal assessments of lateral ventricle volumes in schizophrenia patients indicate that ventricular enlargement is already evident in first-episode psychosis, does not correlate with illness duration, and does not appear to be progressive
(1,
2) . However, contrary to these early CT studies, which were limited by low spatial resolution, small sample sizes, and reliance on a single measure of brain volume, the growing number of longitudinal magnetic resonance imaging (MRI) studies
(3 –
11) provide increasingly convergent evidence for progressive brain volume changes during the lifelong course of schizophrenia. Furthermore, most cross-sectional MRI studies (although not all
[12] ) investigating the relationships between brain volumes and illness duration
(13) or comparing brain volumes between recently ill patients and chronically ill patients
(14) also suggest that there are ongoing brain volume changes in schizophrenia patients.
Although longitudinal and cross-sectional MRI studies of schizophrenia patients during the past decade provide compelling evidence of progressive volumetric changes in the frontal and temporal lobes following illness onset, the mechanisms underlying ongoing frontotemporal tissue volume reductions and CSF volume expansions are unclear and are intensely debated
(15,
16) . What factors might mediate progressive brain volume decrement in schizophrenia patients? How important are these changes in the clinical management of patients? Potential causes of brain volume changes include the pathophysiology of schizophrenia, neuroplasticity secondary to antipsychotic use, impoverished environment, fluid imbalance, nutrition or life style factors, and measurement artifacts. The primary aim of this study was to determine whether a functional gene polymorphism that influences neuroplasticity and has been implicated in schizophrenia may mediate progressive brain volume changes in schizophrenia patients.
Brain-derived neurotrophic factor (BDNF) is a trophic protein that is vital in neurodevelopment as well as in modulating activity-dependent synaptic plasticity among mature neurons, particularly in the hippocampus and neocortex
(17 –
19) . Studies indicate that the methionine (Met) variant of a common single nucleotide polymorphism (rs6265), which produces valine (Val) to Met substitution at codon 66 in the proBDNF protein, is associated with inefficient BDNF trafficking, reduced activity-dependent BDNF release, and poorer hippocampus-mediated memory
(20 –
25) . Furthermore, in previous cross-sectional MRI studies, we and others found that Met allele carriers with schizophrenia had smaller frontal and temporal gray matter volumes than Val homozygotes
(22,
26,
27) .
How might a single nucleotide substitution in the gene encoding the proBDNF protein confer poorer cognitive performance and smaller brain volumes? Similar to other neurotrophins, BDNF is synthesized as a precursor peptide (proBDNF). Like other proneurotrophins, proBDNF is important for proper folding, dimerization, and targeting of the mature BDNF
(28) . During intracellular trafficking through the Golgi network, the proBDNF domain is cleaved, and the remaining mature BDNF protein is packaged into secretory vesicles. When hippocampal neurons
(21) or cerebral cortical neurons
(20) are transfected with BDNF
Met variant, there is less efficient localization of mature BDNF to secretory vesicles compared with the wild-type BDNF
Val . Thus, substitution of valine with methionine in the proBDNF is believed to disrupt folding and dimerization of BDNF and in turn results in defective intracellular protein trafficking and diminished BDNF synthesis
(20,
21) . Additionally, mechanisms by which BDNF
Met variant decreases gray matter volumes may be mediated during neurodevelopment through BDNF’s neurotrophic effects
(23,
29) . In a relatively BDNF-deficient milieu during embryogenesis, fewer neurons survive, and surviving neurons have smaller soma size and diminished dendritic growth
(17) . Alternatively, BDNF
Met variant may further influence gray matter volumes beyond neurodevelopment through modulating neuroplasticity in mature neurons
(18) .
One approach to understanding when BDNF exerts its influence on brain volumes is to examine the relationships between BDNF val66met genotype status and longitudinal changes in brain volumes. If the effects of BDNF occur exclusively during neurodevelopment, one would not expect BDNF genotype to influence longitudinal brain volume changes in adults who are in their third or fourth decade of life. However, if BDNF val66met genotype has significant effects on longitudinal brain volume changes in young adults with schizophrenia, it suggests that BDNF mediates brain volumes beyond the period of neurodevelopment, possibly via BDNF’s role in neuroplasticity
(17,
18,
30) .
Given that the BDNF
Met variant is associated with diminished BDNF trafficking and BDNF release
(20,
21) and that diminished BDNF signaling in mature neurons decreases dendritic arborization
(18), we hypothesize that Met allele carriers (heterozygotes and Met homozygotes) will have greater reductions in gray matter brain volume than Val homozygotes. The primary aim of this study was to determine whether BDNF val66met genotype status mediates progressive brain volume changes in schizophrenia. We also explored two related issues: 1) the association between antipsychotic treatment and progressive brain volume changes, based on evidence involving animals
(31 –
34) and human subjects
(10,
35 –
37) indicating that antipsychotic medications may contribute to brain volume changes, and 2) the effects of BDNF val66met genotype on longitudinal changes in cognition and clinical symptoms.
Method
Participants
Participants were recruited through the ongoing Iowa Longitudinal Study of Recent-Onset Psychoses
(38) . Participants provided written informed consent after receiving a complete description of the study. The study design and method have been described elsewhere
(38) . In brief, at the time of intake into the study, participants with first-episode schizophrenia or schizophrenia spectrum disorders undergo an extensive evaluation, which includes standardized clinical rating scales (such as the Comprehensive Assessment of Symptoms and History
[39] ), a neuropsychological battery, and MRI of the brain. After intake assessment, participants are evaluated at 6-month intervals using longitudinal follow-up versions of the clinical rating scales. At the 2-, 5-, and 9-year assessment and at 3-year intervals thereafter, the extensive assessments performed at intake are repeated, including MRI of the brain and neuropsychological testing.
The 119 participants included in this study (83 were male; 113 were Caucasians, two were African-Americans, and four were Hispanics) were selected from the larger overall sample based on their having 1) a diagnosis of DSM schizophrenia spectrum disorders according to the Comprehensive Assessment of Symptoms and History (107 had schizophrenia, four had schizophreniform disorder, and eight had schizoaffective disorder); 2) undergone at least two brain MRI scans, that is, at intake and one follow-up scan; and 3) available BDNF genotype data. At the initial assessment, the participants’ mean age was 26.4 years (SD=6.77), and the sample had minimal prior treatment: 50 participants were neuroleptic naive, and the mean duration of antipsychotic treatment for those who had been treated was 9.2 months (SD=17.0). For those who had more than one follow-up MRI scan, the first available scan was used to reduce heterogeneity in duration of follow-up (mean=2.96 years, SD=1.56).
MRI Acquisition and Image Processing
MR images of the whole brain were obtained on a 1.5-T GE (General Electric Medical Systems, Milwaukee) Signa MR scanner. Three different MR sequences were acquired for each participant (T
1 -weighted spoiled gradient-recall acquisition in the steady state, proton density, and T
2 -weighted images). The images were processed using the locally developed software package BRAINS (Brain Research: Analysis of Images, Networks, and Systems; Iowa City, Mental Health Clinical Research Center, University of Iowa Hospitals and Clinics). The imaging parameters as well as detailed descriptions of image analysis methods have been reported elsewhere
(40,
41) . In brief, the T
1 -weighted images were spatially normalized and resampled so that the anterior-posterior axis of the brain was realigned parallel to the anterior-posterior commissure line, and the interhemispheric fissure was aligned on the other two axes. The T
2 -weighted and proton density images were aligned to the spatially normalized T
1 -weighted image using an automated image registration program. These images were then subjected to a linear transformation into standardized stereotaxic Talairach atlas space to generate automated measurements of frontal, temporal, parietal, and occipital lobes, cerebellum, and subcortical regions
(40) . To further classify tissue volumes into gray matter, white matter, and CSF, we used a discriminant analysis method of tissue segmentation based on automated training class selection that utilized data from the T
1 -weighted, T
2 -weighted, and proton density sequences
(41) . In this study, we examined six brain-volume measures: frontal lobe gray matter, temporal gray matter, parietal gray matter, occipital gray matter, lateral ventricles, and sulcal CSF.
Neurocognitive and Symptom Assessments
Participants underwent a comprehensive battery of neuropsychological tests administered by psychometrists who were trained in standardized assessment and scoring procedures. Testing generally took approximately 4 hours to complete, and when necessary, it occurred over several sessions. In order to provide comprehensive yet efficient assessment of the relationships between cognitive performance and BDNF polymorphism, 27 neuropsychological test scores were grouped into cognitive domains on the basis of a priori theoretical considerations
(42,
43) . These five domains were verbal memory, processing speed/attention, problem solving, language skills, and visuospatial skills. These theoretical groupings of cognitive domains have good internal consistency, and their internal reliability has been tested using Cronbach’s alpha
(42,
44) ; the median alpha for the five cognitive domains was 0.80 (range=0.75–0.85). Before cognitive domain scores were derived, the raw test score from each of the neuropsychological test variables was converted to a z score (mean=0, SD=1) based on test performance of the sample at intake assessment. Scores were reversed where necessary so that a larger negative score indicates poorer performance below the mean. From these z scores, each domain score is the summed average of its component neuropsychological test variables.
Using the Scale for the Assessment of Positive Symptoms (SAPS) and Scale for the Assessment of Negative Symptoms (SANS)
(39), symptom severity at the time of intake and follow-up was assessed along three domains: psychotic, negative, and disorganized. The psychotic domain was defined as the sum of the global ratings for hallucinations and delusions in the SAPS; the disorganized domain was the sum of SAPS global ratings for positive formal thought disorder, bizarre/disorganized behavior, and inappropriate affect; and the negative domain was defined as the sum of the SANS global ratings for affective flattening, alogia, avolition/apathy, and anhedonia/asociality. The maximum possible scores for the psychotic, disorganized, and negative domains are 10, 15 and 20, respectively, with higher scores indicating more severe symptoms.
Genetic Analyses
DNA was prepared by high-salt extraction from whole blood. BDNF val66met genotyping was performed using the fluorogenic 5′ nuclease method (TaqMan, Applied Biosystems, Foster City, Calif.) with reagents obtained from Applied Biosystems, including VIC- and FAM-labeled probes and TaqMan Universal PCR (polymerase chain reaction) Master Mix. PCR and allele calling were performed on a StrataGene Mx3000P qPCR thermocycler. Replicate samples were included on all genotyping plates to ensure accurate allele calling.
Statistical Analyses
In neuronal cell cultures, inefficient BDNF trafficking is observed when the Met variant is expressed alone or when Met and Val variants are coexpressed
(20,
21) . Therefore, since Met-allele-carrier status is associated with impaired BDNF secretion, heterozygotes and Met homozygotes in this study were categorized as “Met group” and compared against Val homozygotes, or “Val group.”
To investigate the effects of BDNF genotype on progressive brain volume changes, repeated-measures analysis of covariance was used to examine whether genotype grouping (Met allele carriers versus Val homozygotes) had significant interactions with longitudinal within-subject changes in each region of interest. In the general linear statistical model for each of the six regions of interest (frontal lobe gray matter, temporal gray matter, parietal gray matter, occipital gray matter, lateral ventricles, and sulcal CSF), intake and follow-up volumes were the dependent measures (within-subject factors). The independent measure was genotype grouping. Total brain compartment volume, age at intake scan, interscan interval, and interscan antipsychotic exposure (dose-years; 1 dose-year=100 mg chlorpromazine equivalent per day for 1 year) were entered as covariates. Total brain compartment volume adjusts for individual differences in overall cranial size. Age at intake scan was used as a covariate since age is known to influence brain volumes. Likewise, interscan interval was also entered as a covariate because of differences in the interval between intake and follow-up scans among the participants. For regions of interest where genotype grouping had significant interactions with within-subject factors of repeated measures in brain volumes (i.e., changes in brain volume over time differed significantly across genotype groupings), changes in these regions of interest over time within each genotype grouping were examined in post hoc analyses with paired t tests.
To explore the effects of BDNF genotype on longitudinal changes in neurocognition and psychopathology, a similar statistical approach of repeated-measures analysis of covariance was used. In the general linear statistical model for each of the five cognitive domain scores (verbal memory, speed/attention, problem solving, language, and visuospatial abilities) and three symptom domain ratings, intake and follow-up scores were the dependent measures (within-subject factors). The independent measure was genotype grouping. Gender, age at baseline assessment, interscan interval, and interscan antipsychotic exposure (dose-years) were entered as covariates. Full-scale IQ was an additional covariate in the analyses of neurocognition.
Lastly, exploratory regression analyses were performed to examine whether the relationships between longitudinal changes in MRI brain volumes and changes in neurocognition were different across BDNF genotype groupings.
Results
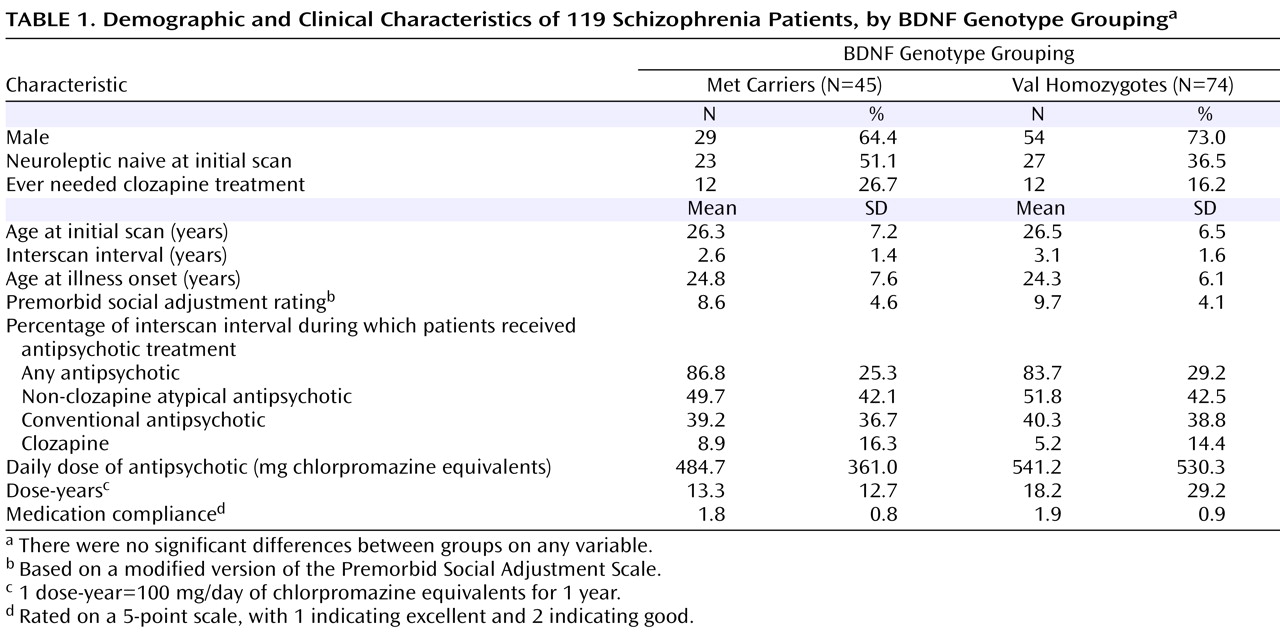
Genotype (Met/Met=3.4%, Val/Met=34.5%, Val/Val=62.1%) and allele (20.6% Met) frequency distributions were consistent with those expected in Caucasian samples. Met and Val groups did not differ significantly in age, interscan interval, or factors associated with poor outcome in schizophrenia—male gender, early age at illness onset, and poor premorbid social adjustment (
Table 1 ).
At the baseline scan, 50 participants were neuroleptic naive. The proportions of neuroleptic-naive patients did not differ significantly between BDNF genotype groupings. Because this was a long-term longitudinal study, antipsychotic treatment during the interscan interval was naturalistic. Patients received antipsychotic treatment for much of the interval between scans (mean=84.9% of the interscan interval [SD=27.7]). Non-clozapine atypical antipsychotics were the predominant class of antipsychotics. Twenty-four patients (20.2%) required clozapine treatment during the interscan interval. The mean daily antipsychotic dose for the sample was 519.8 mg chlorpromazine-equivalent (SD=472.6). Compliance with antipsychotic treatment ranged from good to excellent (mean=1.87 [SD=0.86] on a 5-point scale, with 1 indicating excellent and 2 indicating good). None of these measures of antipsychotic treatment differed significantly between BDNF genotype groupings (
Table 1 ).
Longitudinal Changes in MRI Brain Volumes Between BDNF Genotype Groupings
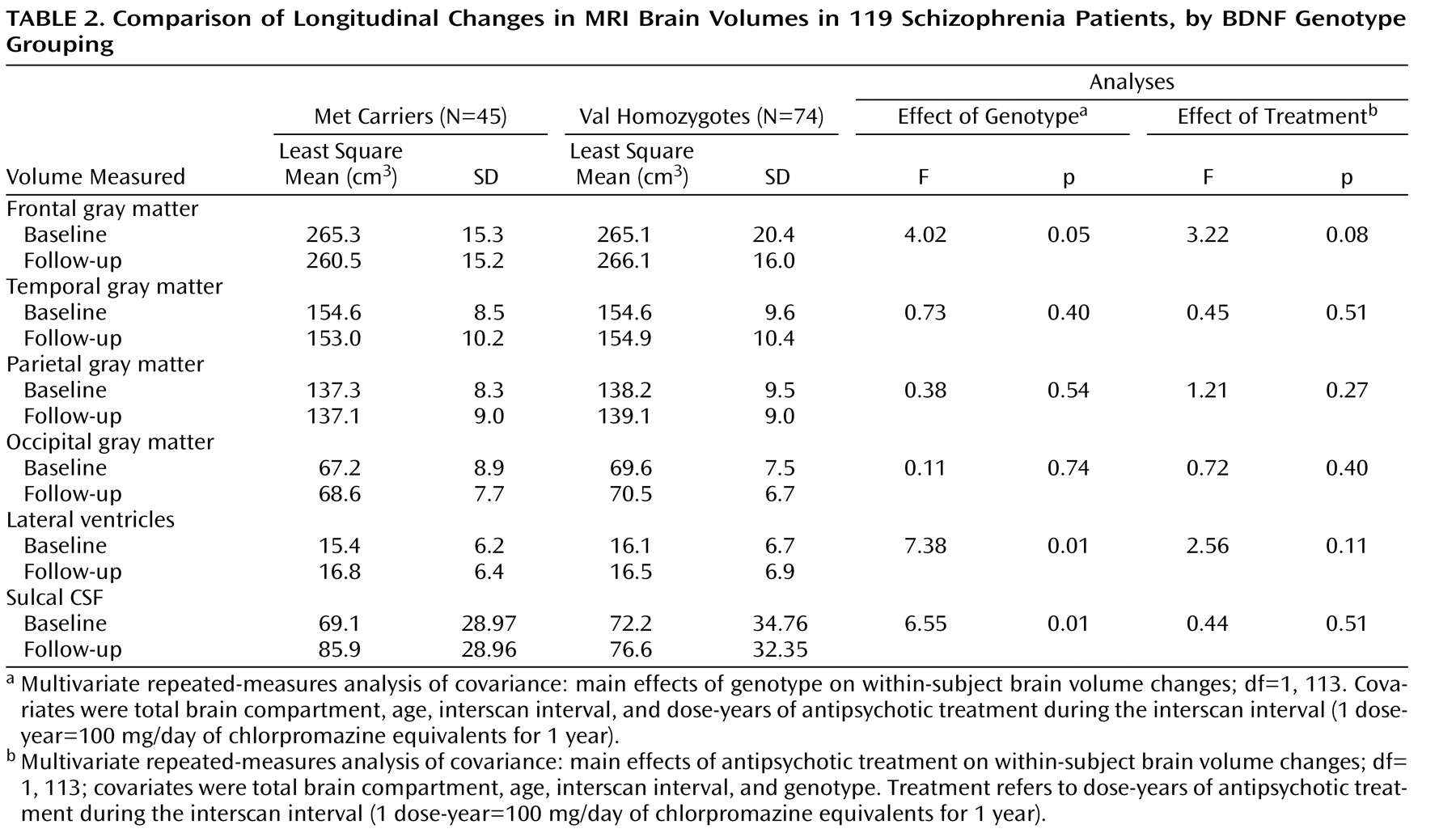
Least square means for MRI brain volumes at baseline and at follow-up assessment are summarized in
Table 2 . There was a significant genotype main effect on within-subject changes in frontal gray matter volume (F=4.20, df=1, 113, p=0.05). Met allele carriers showed significant reductions in frontal gray matter volume at follow-up assessment compared with baseline (mean=–4.83 cm
3 ; t=2.08, df=44, p=0.04). Follow-up frontal gray matter volume did not differ significantly from baseline among Val homozygotes (mean=–0.98 cm
3 ; t=0.56, df=73, p=0.58). There were no significant genotype main effects on within-subject changes in temporal, parietal, or occipital gray matter volumes.
Significant genotype main effects were also observed on within-subject changes in the volumes of the lateral ventricles and sulcal CSF (see
Table 2 ). Compared with baseline volumes, Met allele carriers had significant volume increases in the lateral ventricles and sulcal CSF at follow-up assessment (mean=1.30 cm
3 or 16.12 cm
3, respectively; t≥3.95, df=44, p≤0.0003). Within-subject changes in these CSF volumes were not statistically significant in Val homozygotes. Furthermore, differential changes in sulcal CSF volumes between genotype groupings were restricted to the frontal and temporal lobes. Significant genotype main effects were found on within-subject changes in frontal and temporal CSF volumes (F≥6.71, df=1, 113, p≤0.01), but not in parietal or occipital CSF volumes (F≤1.84, df=1, 113, p≥0.18). Met allele carriers again showed greater volume increases in frontal CSF (mean=11.94 cm
3 ) and temporal CSF (mean=1.63 cm
3 ) than did Val homozygotes (mean=4.69 cm
3 and –0.47 cm
3, respectively).
Interrelationships of Antipsychotic Treatment During Interscan Interval With Brain Volume Changes and BDNF Genotype Groupings
To examine the interrelationships between antipsychotic treatment and BDNF genotype on longitudinal MRI brain volume changes, antipsychotic exposure during the interscan interval (in dose-years) was included as a covariate in the repeated-measures general linear models (
Table 2 ). For frontal gray matter volume changes, the antipsychotic treatment main effect approached but did not reach statistical significance (F=3.22, df=1, 113, p=0.08). There were no statistically significant antipsychotic treatment main effects on within-subject changes in the other MRI brain volume measures either.
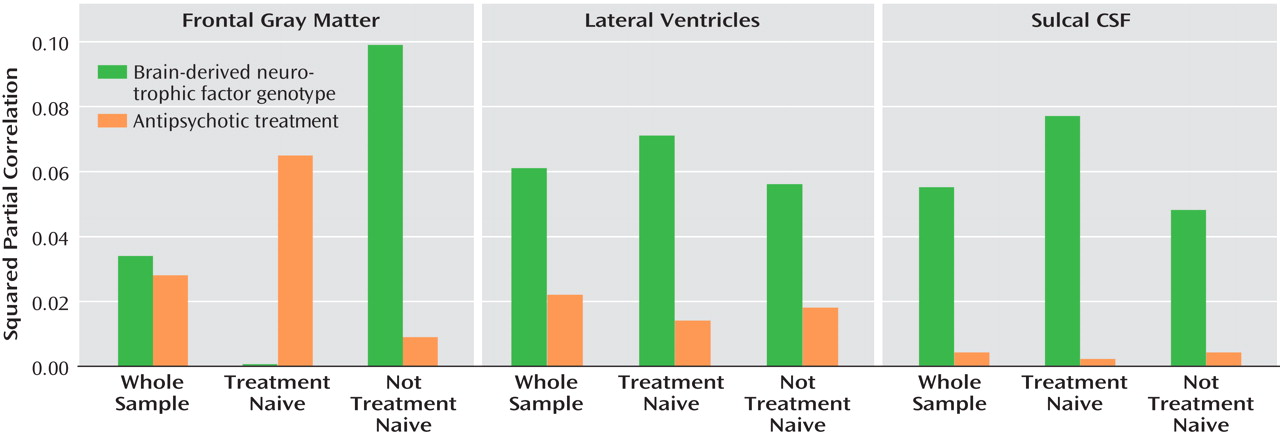
Additionally, for brain regions where there were significant repeated-measures within-subject genotype main effects (i.e., frontal gray matter, lateral ventricles, and sulcal CSF), we used regression analyses to explore the effect sizes and relative independent contributions from BDNF genotype and from antipsychotic exposure on variance in longitudinal brain volume changes. Type II squared partial correlations between brain volume changes and genotype grouping and between brain volume changes and treatment are summarized in
Figure 1 .
BDNF genotype accounted for 3.4% (t=2.01, df=1, 118, p=0.05) of the variance in frontal gray matter volume changes, while antipsychotic treatment contributed an additional 2.8% (t=1.79, df=1, 118, p=0.08). Higher dose-years correlated significantly with reductions in greater frontal gray matter volume (Spearman partial r=–0.20, df=117, p=0.03). However, these interrelationships varied according to antipsychotic treatment status at baseline scan.
For patients who were neuroleptic naive at baseline scan, antipsychotic treatment accounted for 6.5% (t=1.75, df=1, 118, p=0.09) of the variance in frontal gray matter changes, while BDNF genotype had no independent contribution (squared partial r=0.00; t=0.05, p=0.96). Higher dose-years correlated significantly with greater reductions in frontal gray matter volume (Spearman partial r=–0.32, df=48, p=0.03). Conversely, among patients who were already receiving antipsychotic treatment at the time of the baseline scan, the relative independent contributions to variance in frontal gray matter volume changes were 9.9% from BDNF genotype (t=2.63, df=1, 118, p=0.01) and 0.9% from antipsychotic treatment (t=0.76, df=1, 118, p=0.45).
The variances in volume changes in the lateral ventricles and sulcal CSF accounted for by BDNF genotype were larger (∼6%) than those accounted for by antipsychotic treatment (≤2%; see
Figure 1 ). These interrelationships did not differ substantially according to antipsychotic treatment status at baseline scan.
Longitudinal Changes in Neurocognition and Symptom Severity Between BDNF Genotype Groupings
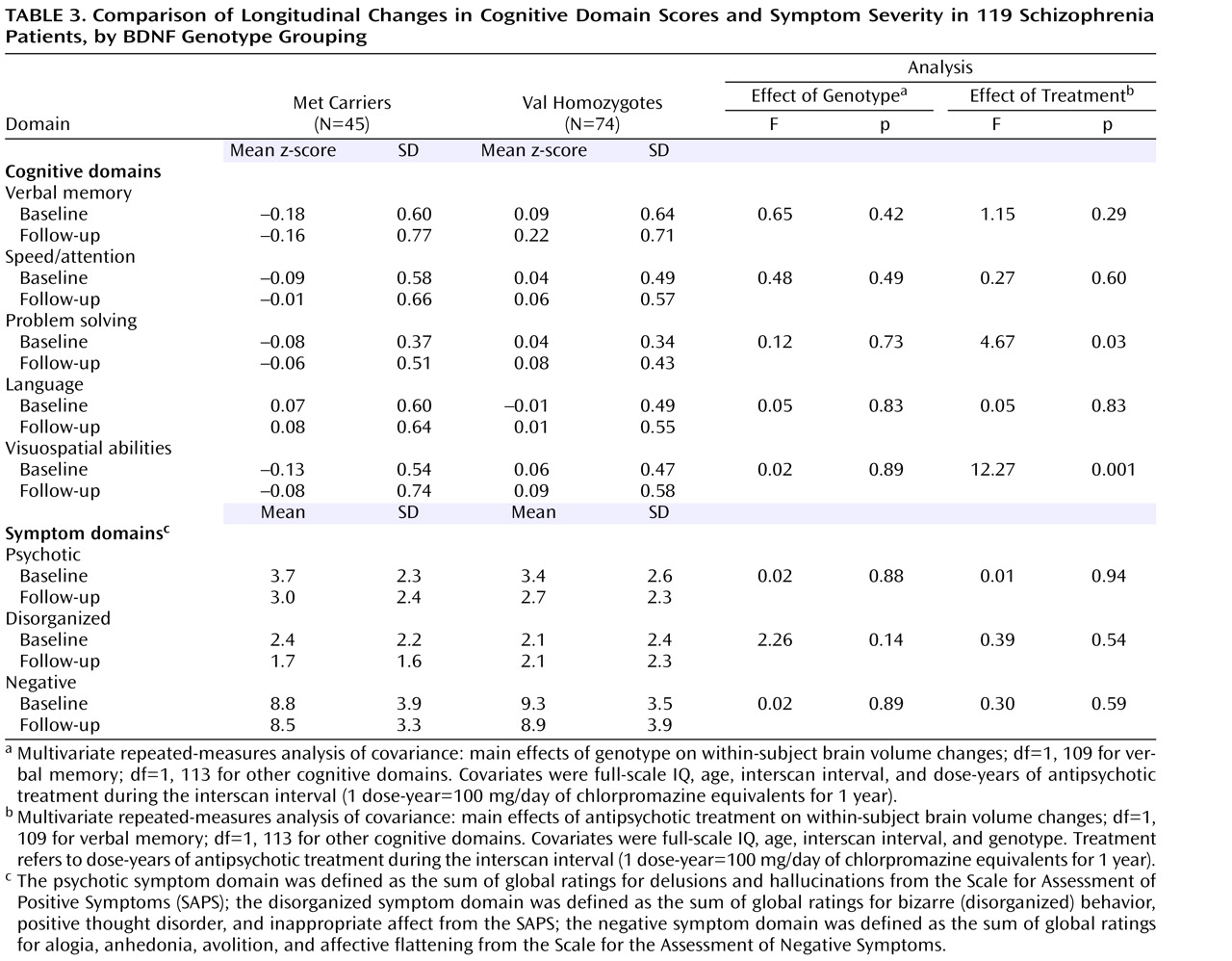
Least square means for cognitive domain scores and symptom severity at baseline and at follow-up assessment are summarized in
Table 3 . Each of the five cognitive domain scores had improved significantly at follow-up (within-subject time main effect; F≥8.19, df=1, 109 or 1, 113, p≤0.005). Negative symptoms at follow-up were also significantly less severe than at baseline (within-subject time main effect; F=4.18, df=1, 114, p=0.04). The severity of psychotic and disorganized symptoms was lower at follow-up, although the difference approached but did not achieve statistical significance (within-subject time main effect; F≤3.53, df=1, 114, p≥0.06).
However, none of these longitudinal changes in neurocognition or in symptom severity differed significantly between genotype groupings. There were no significant genotype main effects on repeated measures within-subject changes in cognitive domain scores or in symptom severity (
Table 3 ). Antipsychotic treatment received during the interscan interval had no significant main effects on within-subject changes in symptom severity or cognitive performance, except on problem solving and visuospatial abilities domain scores (F≥4.67, df=1, 113, p≤0.03; partial r≥–0.20, df=118, p≤0.03).
There were significant between-subject genotype main effects on domain scores for verbal memory, problem solving, and visuospatial abilities (F≥4.19, df=1, 109 or 1, 113, p≤0.04). Met allele carriers had greater impairment than Val homozygotes on these cognitive domains (univariate genotype main effects on baseline verbal memory domain score [F=4.65, df=1, 114, p=0.03], follow-up verbal memory [F=6.44, df=1, 114, p=0.01], baseline problem solving [baseline: F=3.04, df=1, 117, p=0.08], follow-up problem solving [F=2.85, df=1, 117, p=0.09], baseline visuospatial abilities [F=4.53, df=1, 117, p=0.03], and follow-up visuospatial abilities [F=2.49, df=1, 117, p=0.12]). Between-subject genotype main effects on speed/attention and language domain scores were not statistically significant. Symptom severity also did not differ significantly between genotype groupings.
Differential Effects of BDNF Genotype on the Relationships Between Frontal Gray Matter Volume Changes and Longitudinal Changes in Neurocognition
Since Met allele carriers showed greater reductions in frontal gray matter volume, we explored how longitudinal changes in this volume may be related to changes in neurocognition and whether such relationships differed between genotype groupings. We first tested the overall effect of frontal gray matter volume change scores on all five cognitive domain change scores in a joint omnibus multivariate regression test. Frontal gray matter volume change (follow-up volume minus baseline volume) was the independent variable, and the five cognitive domain change scores were entered simultaneously as dependent variables. Covariates in the joint omnibus test were total brain compartment volume, age at intake scan, interscan interval, and dose-years of antipsychotic. A frontal gray matter volume change-by-genotype interaction term was also entered in the regression model.
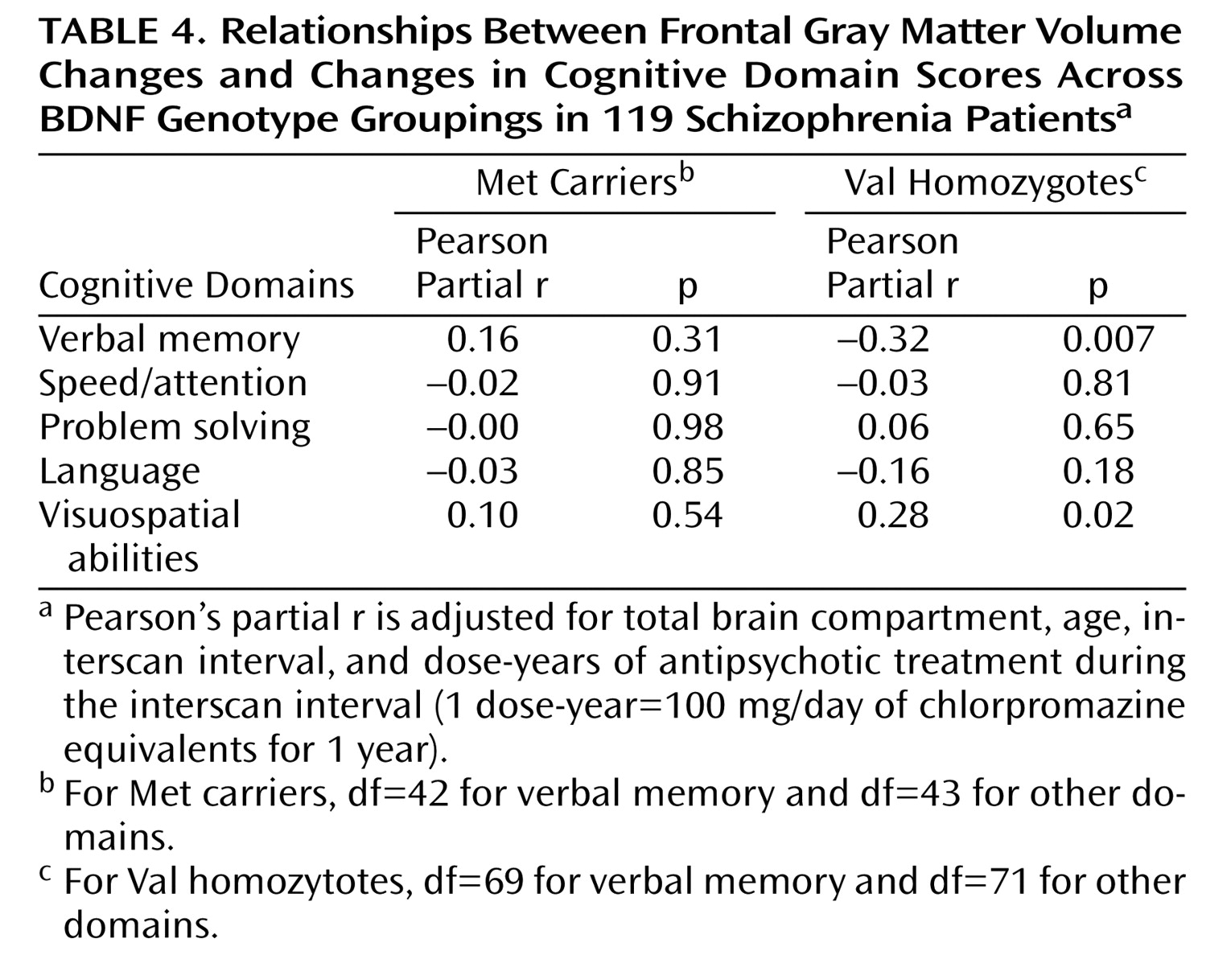
In the joint omnibus test, there was a significant overall main effect of frontal gray matter volume change on longitudinal changes in neurocognition (F=2.70, df=5, 103, p=0.02). The frontal gray matter volume change-by-genotype interaction term was also statistically significant (F=3.75, df=5, 103, p=0.004), which indicated that the relationships between frontal gray matter volume changes and changes in cognitive domain scores differed between genotype groupings. Pearson partial correlation coefficients for the relationships between frontal gray matter volume change scores and cognitive domain change scores within each genotype group are summarized in
Table 4 .
Among Val homozygotes, increases in frontal gray matter volume were significantly associated with worsening verbal memory performance (
Table 4 ; see also supplementary
Figure 1 a in the data supplement that accompanies the online version of this article). In Met allele carriers, decreases in frontal gray matter volume correlated with greater decline in verbal memory performance, although the correlation was not statistically significant. Post hoc univariate analyses showed significant frontal gray matter volume change-by-genotype interaction effects on verbal memory change scores (F=8.52, df=1, 114, p=0.004).
Among Val homozygotes, decreases in frontal gray matter volume correlated significantly with worsening of visuospatial abilities (see
Table 4 ; see also supplementary Figure 1b in the online data supplement). Although the relationships between frontal gray matter volume and visuospatial abilities were weaker in Met allele carriers, the association was in the same direction as that observed in Val homozygotes. For speed/attention, problem solving, and language cognitive domains, there were no significant correlations between changes in frontal gray matter volume and changes in cognitive domain scores (see
Table 4 ).
Discussion
In this study, we investigated the effects of BNDF val66met genotype on longitudinal changes in MRI brain volumes, neurocognition, and symptom severity early in the course of schizophrenia. We found that Met allele carriers had greater reductions in frontal gray matter volume as well as greater increases in the volume of the lateral ventricles and sulcal (especially frontal and temporal) CSF than Val homozygotes. Although the contributions from BDNF genotype and antipsychotic treatment to progressive reductions in frontal gray matter volume were similarly modest in the overall sample, their relative effects appear to vary depending on antipsychotic treatment status at intake. Even though Met allele carriers had poorer verbal memory and visuospatial abilities than Val homozygotes, longitudinal changes in cognition or in symptom severity did not differ between BDNF genotype groupings. Finally, our study suggests that the relationships between frontal gray matter changes and changes in cognition may be different across BDNF genotypes, particularly with respect to verbal memory.
That Met allele carriers showed progressive reductions in frontal gray matter and larger expansions of CSF volume than Val homozygotes is consistent with recent cross-sectional MRI studies
(22,
26) reporting associations between BDNF
Met variant and smaller frontal and temporal gray matter volumes. The mechanisms by which BDNF
Met variant affects brain volumes may be mediated via BDNF’s neurotrophic effects during neurodevelopment
(30) . Since BDNF
Met variant is associated with inefficient intracellular BDNF trafficking and reduced BDNF secretion
(20,
21), the resultant BDNF-deficient milieu during neurodevelopment may lead to diminished neuronal proliferation, fewer neurons, small soma size, and reduced dendritic growth
(17), and in turn smaller gross brain volumes measured using in vivo MRI. Our current findings of progressive brain volume changes among schizophrenia patients in their mid-20s to early 30s suggest that BDNF
Met variant may also influence brain plasticity in young adults. Since BDNF
Met variant reduces activity-dependent BDNF release
(20,
21) and diminished BDNF signaling in mature neurons results in decreased dendritic arborization and neuronal loss
(18), the progressive gross brain volume changes observed in this study may represent ongoing neuroplastic effects of BDNF.
Although our study suggests that BDNF val66met genotype status may be one of the factors mediating progressive brain volume changes after illness onset in schizophrenia, potential confounders need to be considered. Both genotype groupings were comparable with regard to well-replicated predictors of poor outcome in schizophrenia (i.e., poor premorbid social adjustment, early age at illness onset, and male gender). Thus, the association between BDNF Met variant and progressive brain volumetric deficits in schizophrenia appears to be unrelated to such predictors of poor outcome.
Another potential confounding factor is antipsychotic treatment. Long-term exposure to antipsychotics in monkeys has been shown to reduce fresh brain weights and volumes by ∼10% for both haloperidol and olanzapine
(31) . It has been suggested that these antipsychotic-induced brain volume reductions may be related to reduced glial cell production and to decreased dendritic arborization and dendritic spine density
(32) . In this study, we found that BDNF genotype and antipsychotic treatment each independently accounted for approximately 3% of the variance in frontal gray matter changes for the whole sample. Greater antipsychotic treatment correlated with greater frontal gray matter volume reduction. However, the nature of this association cannot be satisfactorily resolved in a naturalistic study such as this. More antipsychotic treatment may simply be an indicator of illness severity. That is, patients with more severe forms of schizophrenia may not only receive higher doses of antipsychotics but also exhibit greater progressive reductions in frontal gray matter volume because of the more deteriorative nature of their illnesses. However, when viewed in conjunction with reduced fresh brain weights and volumes in monkeys that have received chronic treatment with haloperidol or olanzapine
(31,
32), our findings do suggest that antipsychotic treatment may contribute to progressive reductions in frontal gray matter volume in patients with schizophrenia.
The relative prominence of antipsychotic treatment effects among initially neuroleptic-naive patients further supports the view that antipsychotics may play a role in mediating progressive frontal gray matter loss. In patients who were neuroleptic naive at intake, antipsychotic treatment accounted for ∼7% of the variance in frontal gray matter volume reductions. On the other hand, the effects of antipsychotic treatment on frontal gray matter volume reductions were comparatively smaller (∼1%) in patients who were already receiving antipsychotic treatment by the time of the intake scan. Among these initially non-antipsychotic-naive patients, most of the frontal gray matter volume reductions related to antipsychotic treatment may have already occurred prior to the intake scan, such that any additional frontal gray matter reductions during the interscan interval were predominantly associated with BDNF Met-allele-carrier status. This differential predominance of BDNF genotype and antipsychotic treatment effects on the variance in frontal gray matter volume reductions between the two subgroups of patients in this study clearly requires replication. Controlled treatment studies with first-episode and chronic schizophrenia patients are needed to further clarify the relative contributions of BDNF genotype and antipsychotic treatment of progressive brain volume changes.
What are the clinical implications of these findings? Does reduction in frontal gray matter volume correlate with greater decline in cognition, and in particular, does such a relationship affect Met allele carriers differently? In the joint omnibus test assessing the overall relationship between brain volume and cognition, we found that frontal gray matter volume changes had significant effects on changes in cognition. Specifically, reductions in frontal gray matter volume were associated with less improvement in visuospatial abilities regardless of BDNF genotype status. The brain volume-cognition relationships between BDNF genotypes for verbal memory are more complex. Frontal gray matter volume reductions correlated nonsignificantly with greater decline in verbal memory performance among Met allele carriers. As a group, Met allele carriers showed greater frontal gray matter volume reductions and had poorer verbal memory performance than Val homozygotes even though longitudinal changes in verbal memory scores were no different from cognitive changes seen in Val homozygotes. On the other hand, increases in frontal gray matter volume were significantly associated with worsening verbal memory performance among Val homozygotes. Val homozygotes did not show significant longitudinal changes in frontal gray matter volume or in verbal memory performance. The clinical significance of these divergent frontal gray matter-verbal memory relationships across BDNF genotype groupings remains unclear and may have been more evident if we had similar frontal gray matter-verbal memory relationships from healthy volunteers for comparison. Future studies should seek to clarify the associations between brain volume and cognitive changes across BDNF genotype among healthy individuals without schizophrenia.
Even though our findings implicate antipsychotic treatment as a contributory factor for progressive frontal gray matter volume reductions in schizophrenia, and frontal gray matter volume reductions correlate with greater cognitive decline, it would be premature to suggest changing the current status of antipsychotics as the mainstay treatment for schizophrenia. There is a substantial body of literature supporting the efficacy of antipsychotics in ameliorating psychotic symptoms and in reducing the risk of symptom recurrence in schizophrenia
(45) . Recurrence of psychotic symptoms often causes severe disruptions to the lives of patients and family members and can have devastating consequences. Minimizing the risk of psychotic relapses through maintenance antipsychotic treatment remains an important goal in the long-term treatment of patients with schizophrenia. Although this class of drugs may be the best we currently have to offer patients, findings from recent large-scale clinical trials reiterate the limitations of antipsychotics in the management of schizophrenia
(46,
47) . Besides targeting psychotic symptoms, major efforts are already under way to address the current lack of available treatments for cognitive deficits and negative symptoms of schizophrenia
(48,
49) . However, beyond antipsychotics, procognition agents, and treatments for negative symptoms, there is a further need for interventions that target the fundamental pathophysiological processes underlying schizophrenia, including therapies that limit progressive brain volume reductions. A major challenge in developing novel treatments for schizophrenia is our current understanding of the factors mediating progressive neural changes in schizophrenia. Nonetheless, if putatively BDNF-deficient Met allele carriers show greater reductions in frontal gray matter volume, pharmacological strategies that increase BDNF bioavailability
(50) and enhance the neuroplastic effects of BDNF could potentially begin to address the currently unmet need for interventions that curb brain volume reductions in schizophrenia.