The hypothalamic-pituitary-adrenal axis is a major stress response system that is critical for survival and adaptation and is dysregulated in psychiatric disorders such as depression and posttraumatic stress disorder (PTSD)
(1) . The “downstream” brain circuits of the hypothalamus have been well mapped, but the “upstream” brain circuits that are responsible for psychological activation of the hypothalamic-pituitary-adrenal axis in humans remain largely unknown. Relevant pathways likely involve cortical-limbic circuits that detect stimulus salience (e.g., novelty, uncontrollability, social threat) and interpret meaning based on past experience
(2) . Disruptions in cortical-limbic interactions are likely involved in the pathophysiology of psychiatric disorders that are accompanied by hypothalamic-pituitary-adrenal axis abnormalities
(3,
4) . Thus, paradigms in which cortical control of hypothalamic-pituitary-adrenal activity can be studied may illuminate the neural dysregulation underlying these disorders.
Existing evidence shows that the prefrontal cortex, amygdala, hippocampus, and bed nucleus of the stria terminalis are critical cortical-limbic structures that control the hypothalamic-pituitary-adrenal axis
(2) . In previous studies on rodents and primates, the prefrontal cortical regions (e.g., anterior cingulate, prelimbic and infralimbic cortices) have been implicated in the inhibition of hypothalamic-pituitary-adrenal axis activity
(5), modulation of amygdala responses, and facilitation of extinction
(6) . However, these studies did not provide extensive insight into the cortical control of stress systems in humans, since the cortical homology between rodents and primates is limited. Human data utilizing pathological states have also supported the role of hypothalamic-pituitary-adrenal activation in the human amygdala
(7) and an inhibitory role of the frontal cortex
(8) . Additionally, there is growing realization that the hypothalamic-pituitary-adrenal axis and its “end-product” cortisol play important modulatory roles in cortical functions. Cortisol modulates cognitive and emotional processing, social appraisal, and decision making
(9) and could directly contribute to symptom formation in psychiatric disorders. Thus, hypothalamic-pituitary-adrenal axis activity may be shaped by psychological inputs, and its products may in turn alter cognitive/emotional processing of subsequent inputs. Such rapid, dynamic modulation of neural activity would likely occur through rapid (likely membranous) effects of glucocorticoids
(10), since established genomic effects are too slow to modulate immediate behavior. However, there is little human data examining specific cortical regions that are modulated by hypothalamic-pituitary-adrenal axis activation and few existing paradigms in which this can be studied.
Neuroimaging offers opportunities to examine the relationships between cortical activity and hypothalamic-pituitary-adrenal axis function in vivo in humans. However, emotion-induction tasks, which are easily imaged, do not reliably activate the hypothalamic-pituitary-adrenal axis
(11) or produce only modest responses
(12), and robust human hypothalamic-pituitary-adrenal/stress models
(11) are difficult to adapt for neuroimaging. Recent reports have linked the plasma adrenocorticotropic hormone to regional cerebral blood flow (rCBF) in the right insula and anterior cingulate cortex
(12), cortisol levels to amygdala metabolism
(13) and to rCBF in the medial frontal cortex
(14), cortisol variability to blood-oxygen-level-dependent signal in the medial prefrontal cortex and amygdala
(15), and salivary cortisol response to rCBF in the medial prefrontal cortex
(16) . However, these studies were limited by between-subject designs and reliance on correlations that involved single scans or a single hypothalamic-pituitary-adrenal measure and did not disentangle the neural activity underlying hypothalamic-pituitary-adrenal activation from the neural activity associated with cortisol feedback.
Patients with sensitized stress responses may offer a unique opportunity for imaging cortical-limbic control of the hypothalamic-pituitary-adrenal axis, potentially illuminating both normal neurocircuitry and pathophysiological processes. In PTSD patients, altered rCBF has been reported in brain regions (amygdala [
3,
17 ], medial prefrontal cortex
[3], and rostral anterior cingulate
[18] ) that have been implicated in hypothalamic-pituitary-adrenal axis regulation. These patients showed exaggerated emotional responses
(19), altered glucocorticoid sensitivity
(20), and altered hypothalamic-pituitary-adrenal responses
(19,
21), and thus provide a potential model for studying the neural correlates of hypothalamic-pituitary-adrenal axis activation and cortisol action.
To examine the neural substrates underlying hypothalamic-pituitary-adrenal axis activity, we analyzed the temporal links between cortical activation and subsequent hypothalamic-pituitary-adrenal axis response and between circulating cortisol levels and subsequent brain activation. To our knowledge, this is the first such report on neuroendocrine covariation data. Group differences in patterns of regional brain activations have been reported previously
(22,
23) . In response to aversive pictures (aversive versus neutral), healthy comparison subjects activated the amygdala, bilateral insula, dorsal and ventral medial prefrontal cortices, and dorsal and rostral anterior cingulate cortices. It has been previously reported that PTSD and combat comparison subjects activated only the dorsal anterior cingulate cortex, and PTSD patients showed less activation of the rostral anterior cingulate cortex than healthy comparison subjects
(22,
23) . In response to traumatic/stressful scripts (traumatic versus neutral), all subjects were reported to have activated the right insula. Healthy comparison subjects also activated the amygdala and deactivated the ventral medial prefrontal cortex. Combat comparison subjects deactivated both the amygdala and ventral medial prefrontal cortex, and PTSD patients deactivated the dorsal rostral anterior cingulate cortex
(22,
23) .
In the present study, we covaried rCBF responses with postscan plasma adrenocorticotropic hormone responses following a series of emotional challenges in voxel-wise analyses in order to investigate cortical-limbic circuits that influence hypothalamic-pituitary-adrenal axis activation (and subsequent plasma adrenocorticotropic hormone) (
Figure 1 ). To investigate cortical regions through which cortisol might modulate subsequent cognitive/emotional processing, we measured the subjects’ plasma cortisol concentrations prior to emotional activation and covaried the data with the subsequent rCBF responses to emotional stimuli. Additionally, we identified a nonoverlapping set of cortical regions potentially involved in hypothalamic-pituitary-adrenal activation or responsive to circulating cortisol levels.
Method
Participants
After complete description of the study to the participants was provided, written informed consent was obtained. The study was approved by the University of Michigan and Ann Arbor Veterans Affairs Healthcare System. Three groups of right-handed, male participants were recruited via newspaper advertisements. The three study groups were Vietnam combat veterans with PTSD (N=16); age-matched, comparison Vietnam combat veterans with similar combat exposure but without PTSD (N=15); and healthy comparison subjects without combat experience (N=15). PTSD diagnosis was established using the Structured Clinical Interview for DSM-IV and Clinician Administered PTSD Scale. Details of the subjects’ characteristics, including psychiatric history and symptom severity, are available in the data supplement (Part A), which accompanies the online version of this article. The subjects’ urine toxicology screens were negative, and no structural abnormalities were present on magnetic resonance imaging (MRI) scans (1.5 T Signa scanner, GE, Milwaukee). Adrenocorticotropic hormone samples were available from 15 PTSD patients, eight noncombat comparison subjects, and 11 combat comparison subjects. Cortisol level samples were available from 14 PTSD patients, 12 noncombat comparison subjects, and 12 combat comparison subjects.
Neuroimaging Procedures
Positron emission tomography (PET) [
15 O]H
2 O neuroimaging procedures were performed using Siemens ECAT EXACT and HR+ scanners as previously described
(22,
23) . Two types of emotional challenge stimuli were used during the imaging experiment: emotional pictures from the International Affective Picture System
(24) and autobiographical script-driven imagery. The autobiographical script-driven imagery was administered as recommended by Pitman et al.
(25), describing either traumatic/stressful or neutral events. A series of 10 emotional challenges were presented during the 2-hour experiment, which were counterbalanced by stimulus type. PET scans (10 to 15 mCi of [
15 O]H
2 O PET given as an intravenous bolus, three-dimensional data acquisition mode in a 60-second frame) were performed immediately after each stimulus presentation and separated by 12 minutes. Subjective emotional responses were assessed after each scan using the modified Positive and Negative Affect Schedule, and blood samples (3 ml) were collected from an indwelling catheter 5 and 10 minutes after each scan (2 minutes preceding the subsequent scan). Plasma adrenocorticotropic hormone and cortisol levels were collected before and after each scan and were assayed using commercial radioimmunoassay kits (PET procedures, emotional-challenge stimuli, and presentation schedules are described in detail in the data supplement [Part A] accompanying the online version of this article).
Data Analyses
PET image preprocessing and analyses were performed using Statistical Parametric Mapping (SPM) 99 (Wellcome Department of Cognitive Neurology, London). The primary analyses involved voxel-wise analyses of covariance (ANCOVAs) of [ 15 O]H 2 O PET activity (“multisubject covariates-only” module in SPM99), with plasma adrenocorticotropic hormone and cortisol used as separate regressors. We examined 1) links between brain activation patterns and acute (5-minute postscan) plasma adrenocorticotropic hormone response as well as 2) the impact of circulating cortisol levels (2-minute prescan) on rCBF responses. Separate analyses of variance (ANOVAs) were conducted using Statistical Package for the Social Sciences to assess whether the emotional challenges produced changes in plasma adrenocorticotropic hormone or cortisol levels.
Results
Subjective Responses and Neuroendocrine Findings
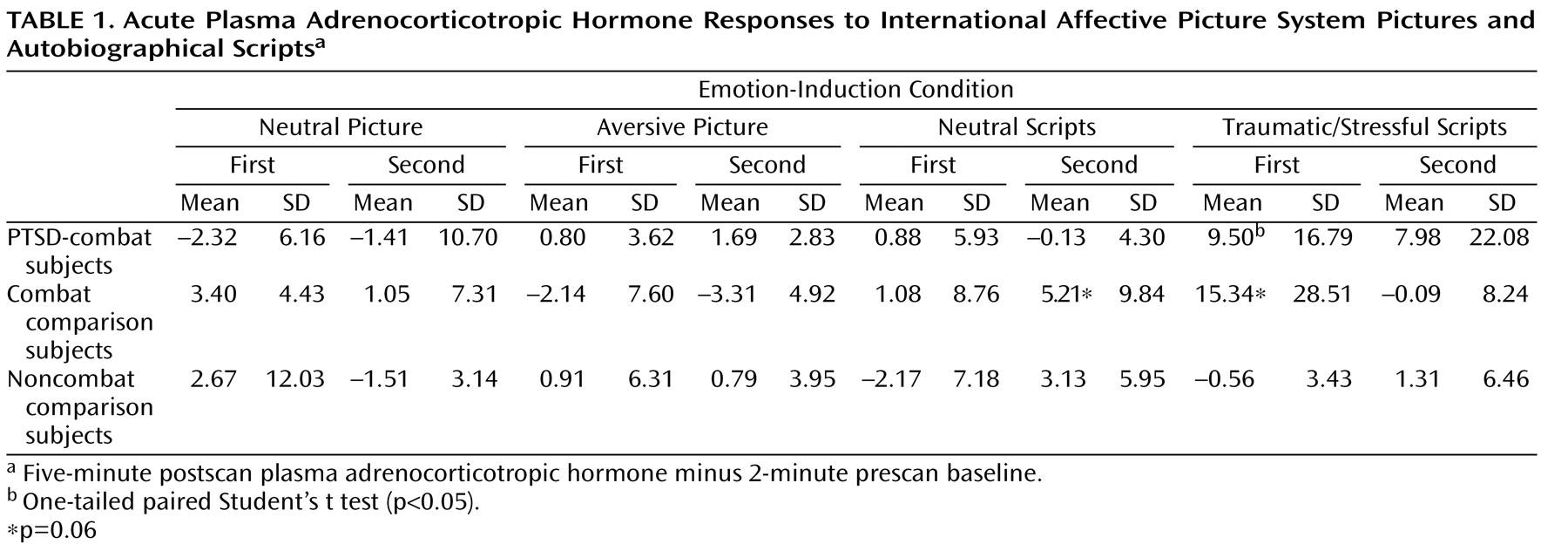
All three study groups reported greater negative emotional response on the Positive and Negative Affect Schedule for aversive versus neutral pictures (F=231.4, df=1, 43, p<0.001) and traumatic/stressful versus neutral scripts (F=121.1, df=1, 41, p<0.001). None of the groups differed in baseline adrenocorticotropic hormone or cortisol levels. Combat-PTSD patients showed significant (p=0.03) acute plasma adrenocorticotropic hormone responses to trauma scripts but not to pictures (
Table 1 ). After removal of a high adrenocorticotropic hormone responder, the combat-exposed healthy comparison group also showed significant (p<0.01) response to trauma scripts. Collection timing was not designed to test the effects of individual stimuli on plasma cortisol; however, cortisol levels were significantly higher during the presentation of scripts compared with the presentation of pictures in combat-PTSD patients (10.3 mg/dl versus 8.2 mg/dl [F=8.19, df=1, 11, p=0.01]) and combat-exposed healthy comparison subjects (9.7 mg/dl versus 8.1mg/dl [F=7.55, df=1, 10, p=0.02]), with no difference in noncombat healthy comparison subjects (see the data supplement [Part B], which accompanies the online version of this article).
Covariation Between Plasma Adrenocorticotropic Hormone and rCBF
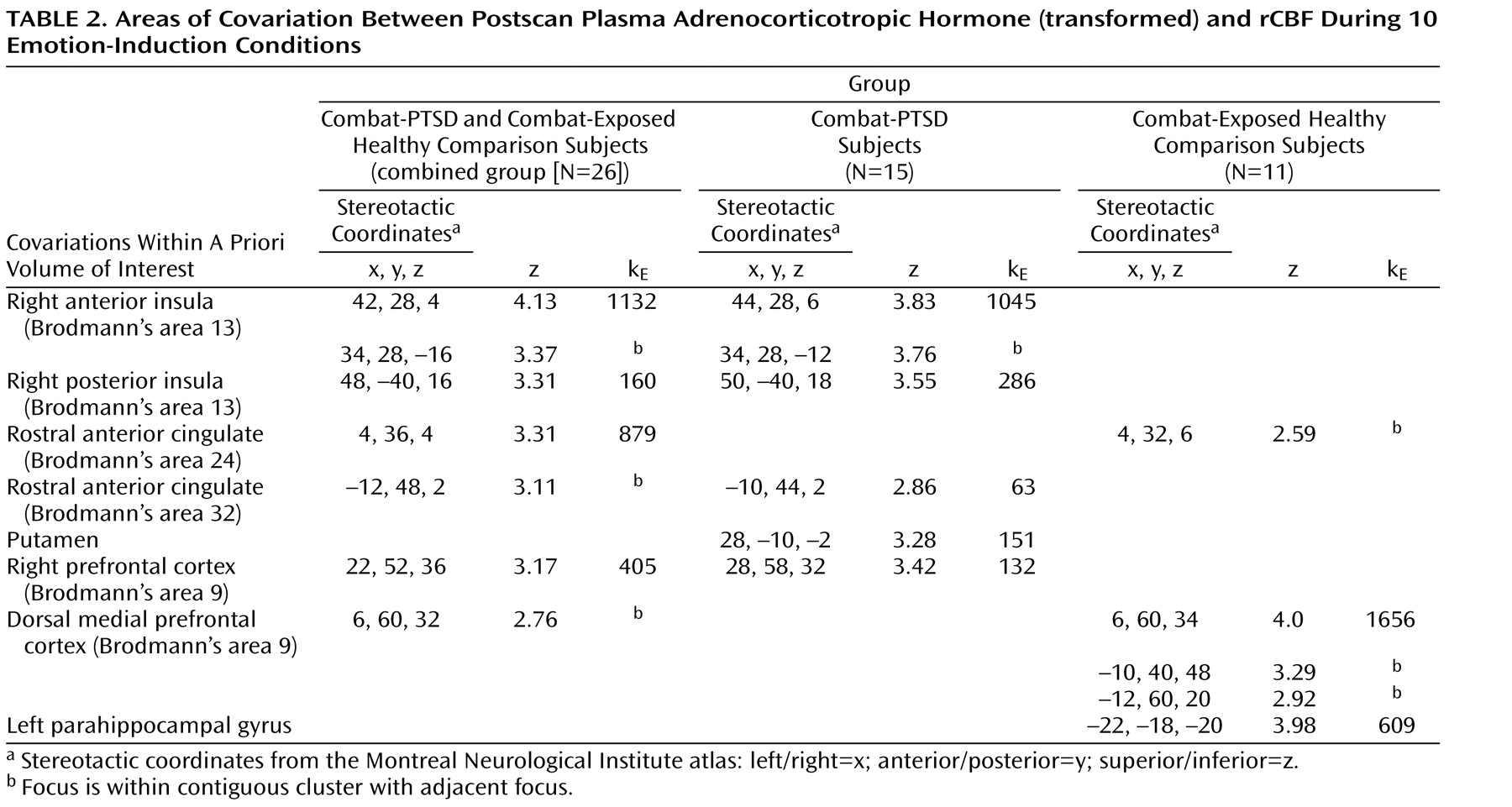
To identify patterns of neural activity reflecting the normative relationship between rCBF responses and subsequent adrenocorticotropic hormone levels, we covaried activity in regions of interest with 5-minute postscan adrenocorticotropic hormone levels. All 10 emotional-challenge scans were used to provide a maximum range of within-subject variance in adrenocorticotropic hormone levels (
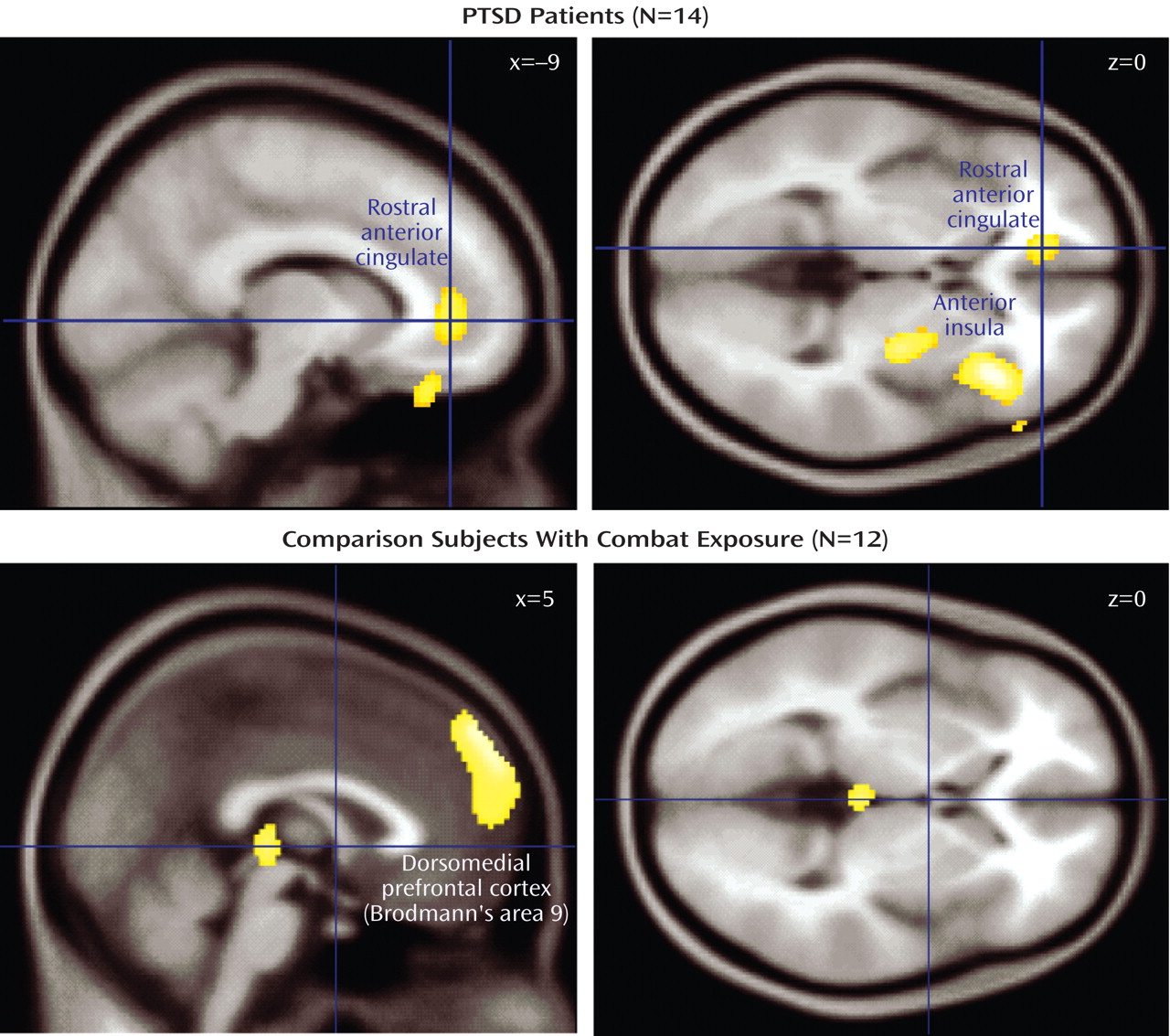
Table 2 ). When all three study groups were combined (N=34), a single large cluster (591 voxels) of positive covariation with adrenocorticotropic hormone levels was found within the a priori area of interest in the right insula ([
38,
28,
2 ] z=3.97), which was significant after small-volume correction (small volume correction, p<0.005) using false discovery rate. A subthreshold peak of covariation was also observed in the dorsal medial prefrontal cortex ([4, 54, 40] z=2.50). No areas of negative covariation were found.
Covariations between brain activity and adrenocorticotropic hormone levels of each study group were then examined separately. In noncombat-exposed healthy comparison subjects (N=8), no significant regions of covariation were found. A detailed presentation of all significant loci of positive adrenocorticotropic hormone covariation among the combat-exposed healthy comparison group is shown in
Table 2 . Since both the combat-PTSD and combat-exposed healthy comparison groups showed meaningful adrenocorticotropic hormone responses to traumatic scripts, they were first analyzed together (N=26) in order to examine the general relationship between cortical activation and rCBF response. Large clusters of covariation were found in the right anterior and posterior insula (Brodmann’s area 13), rostral anterior cingulate cortex (Brodmann’s areas 24, 32) and right and medial prefrontal cortex (Brodmann’s area 9). When the combat-PTSD and combat-exposed healthy comparison groups were analyzed separately in order to identify disease-specific patterns, the combat-PTSD group (N=15) showed adrenocorticotropic hormone covariation in the right anterior and posterior insula (Brodmann’s area 13), rostral anterior cingulate cortex (Brodmann’s area 32), and right prefrontal cortex. The combat-exposed healthy comparison subjects (N=11) showed a large cluster of adrenocorticotropic hormone covariation in the dorsal medial prefrontal cortex (Brodmann’s area 9) and in the parahippocampal gyrus. Because script conditions were the main contributors to the adrenocorticotropic hormone response, we performed a subanalysis for script conditions only to ensure that inclusion of all conditions did not produce spurious results. Despite fewer data points (four scripts versus 10 total challenges), similar patterns of adrenocorticotropic hormone covariation were confirmed in combat-PTSD patients (right anterior insula/inferior frontal gyrus: [36, 38, –14] z=3.99, 402 voxels; right prefrontal cortex: [26, 58, 34] z=3.97, 279 voxels) and in combat-exposed healthy comparison subjects (dorsal medial prefrontal cortex: [10, 62, 22] z=3.40, 94 voxels). Negative covariations were not found in either group.
Covariation Between Plasma Cortisol and rCBF
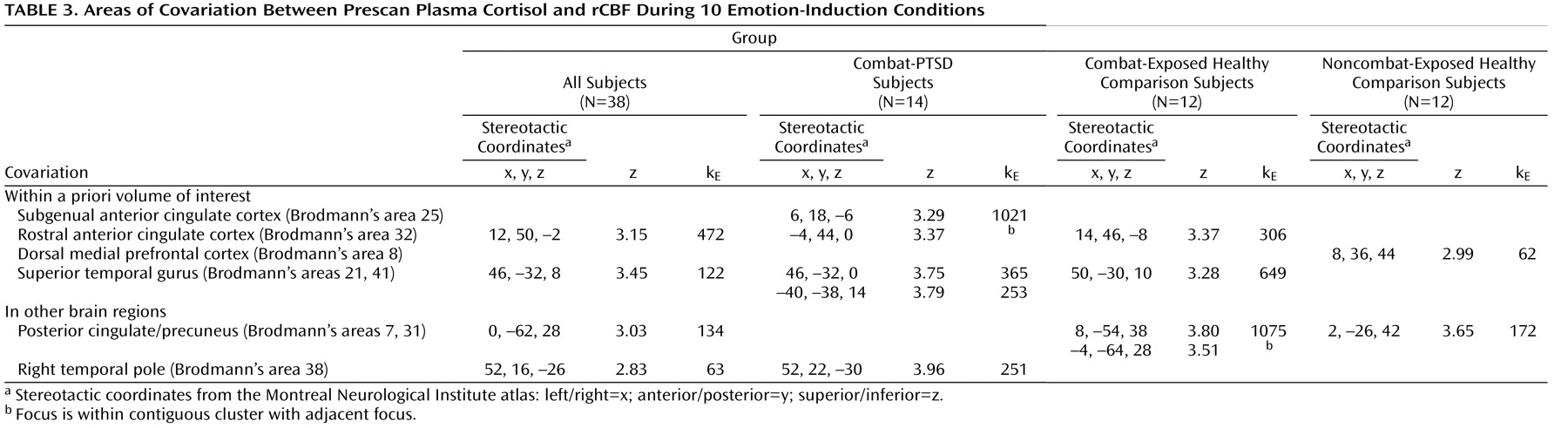
To identify patterns of covariation that might reflect the normative relationship between circulating cortisol and cortical responses, all three groups of subjects were combined in a single analysis of covariation (
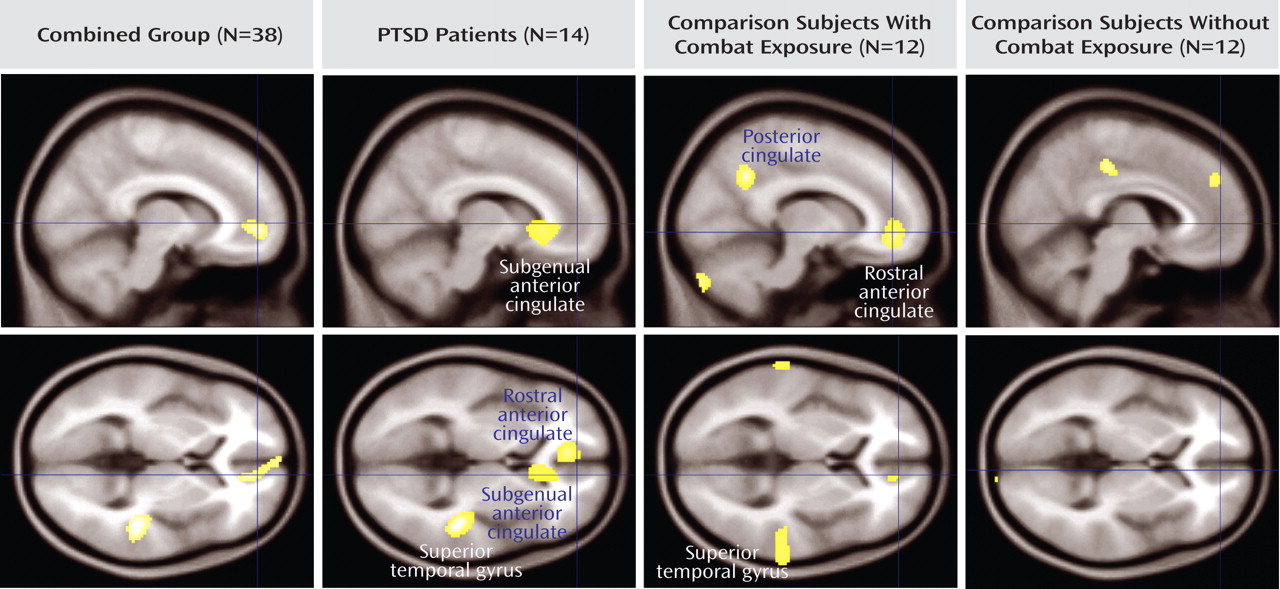
Table 3 ). Prestimulus plasma cortisol levels covaried with rCBF in the rostral anterior cingulate cortex ([12, 50, –2] z=3.12) and superior temporal lobe ([46, –32, 8] z=3.45). To examine potential differences in covariation patterns among combat-PTSD and comparison subjects, similar analyses were performed separately for each group. Negative covariations were not found within the volume of interest. Healthy comparison subjects showed covariation in the dorsal medial prefrontal cortex (Brodmann’s area 8) and outside the volume of interest in the posterior cingulate gyrus (Brodmann’s area 31). Combat-PTSD patients showed covariation spanning the rostral anterior cingulate cortex (Brodmann’s area 32) and subgenual anterior cingulate (Brodmann’s area 25); in the bilateral superior temporal gyrus (Brodmann’s areas 21, 41); and outside the volume of interest in the right temporal pole (Brodmann’s area 38). Combat-exposed healthy comparison subjects showed covariation in the rostral anterior cingulate cortex and outside the volume of interest in the medial and left precuneus (Brodmann’s areas 7, 31). Adding depression severity (Beck Depression Inventory) as a nuisance covariate did not affect any of our peaks.
Discussion
The data presented in the present study demonstrate that adrenocorticotropic hormone responses to emotional stimuli may be modulated by paralimbic and prefrontal activity and that activity in the subgenual anterior cingulate may, in turn, be sensitive to prestimulus plasma cortisol levels. Autobiographic trauma scripts elicited adrenocorticotropic hormone responses in trauma-exposed individuals (with or without PTSD), which were positively correlated with the severity of symptoms in combat-PTSD subjects. In trauma-exposed subjects, adrenocorticotropic hormone covaried with activity in the right insula, rostral anterior cingulate cortex (Brodmann’s areas 25, 32), and dorsal medial prefrontal cortex (Brodmann’s areas 9, 10), with differential patterns in combat-PTSD patients and combat-exposed healthy comparison subjects. This suggests involvement of the insula, rostral anterior cingulate cortex, and dorsal medial prefrontal cortex in “top down” cortical activation or modulation of neuroendocrine stress responses. In turn, preactivation cortisol levels covaried with rCBF responses in the rostral anterior cingulate cortex and right superior temporal gyrus (Brodmann’s area 42) in the combined group (combat-PTSD and combat-exposed and nonexposed healthy comparison groups) and in the subgenual anterior cingulate cortex (Brodmann’s area 25) in combat-PTSD patients, which suggests that activation of these regions may be modulated by circulating levels of cortisol. To our knowledge, these data provide the first demonstration of a methodology that allows in vivo identification of the human neurocircuitry controlling hypothalamic-pituitary-adrenal axis activity and those brain regions modulated by the neuroendocrine milieu.
Cortico-Limbic Circuitry and Adrenocorticotropic Hormone Response
Both general aversive (International Affective Picture System pictures) and trauma-specific (autobiographical scripts) challenges elicited strong emotional responses in all participants, but acute adrenocorticotropic hormone responses were observed only in combat-exposed subjects and only in response to trauma scripts, which supports the notion that the hypothalamic-pituitary-adrenal axis responds to specific, personally salient psychological stimuli rather than to general distress
(26) . Adrenocorticotropic hormone responses were found in both combat-exposed healthy comparison subjects and combat-PTSD patients, suggesting that hypothalamic-pituitary-adrenal sensitization does not necessarily reflect pathological processes. Voxel-by-voxel analyses revealed significant covariation of the right anterior insula, rostral anterior cingulate cortex, and dorsal medial prefrontal cortex activity with subsequent adrenocorticotropic hormone responses in the combat-exposed groups. Trauma-script activation of the insula has been previously reported
(18), but our finding of a dynamic association between insula activity and adrenocorticotropic hormone response is novel and consistent with a previous single photon emission computed tomography study that reported higher insular activity in subjects with higher adrenocorticotropic hormone levels
(12) . This finding is also consistent with the role of the insula in orchestrating negative emotional responses, since the insular cortex has been implicated in processing of aversive emotional stimuli
(27), recall of emotional material
(4,
28), and integration of visceral sensory information and emotional and autonomic responses
(29) . The extensive interconnections of the insula with the amygdala, temporal pole, superior temporal gyrus, and orbitofrontal lobe are also consistent with a role in influencing psychological activation of the hypothalamic-pituitary-adrenal axis
(30) .
The covariation observed between the rostral anterior cingulate cortex activation and adrenocorticotropic hormone responses is consistent with evidence linking rostral anterior cingulate cortex to processing of aversive emotional stimuli
(27,
31) . The rostral anterior cingulate cortex is connected with the amygdala, periaqueductal gray matter, nucleus accumbens, and ventral striatum, and it has also been associated with emotional behavior and autonomic responses in animals
(32) . Additionally, lesions of the anterior cingulate cortex lead to attenuated autonomic responses to conditioned stimuli. The association of activity in the anterior insula and rostral anterior cingulate cortex with subsequent adrenocorticotropic hormone responses is particularly intriguing in light of a recent study that linked both the insula and anterior cingulate cortex responses with subsequent inflammatory measures in asthma patients
(33) . Our findings suggest the possibility that psychogenic, cortically modulated hypothalamic-pituitary-adrenal axis activation may mediate the functional coupling of activity in emotional circuits with peripheral immune function.
Adrenocorticotropic hormone responses were strongly associated with activity in the dorsal medial prefrontal cortex in combat-exposed healthy comparison subjects but not in combat-PTSD patients. In humans, the medial prefrontal cortex has been implicated in emotion regulation and autonomic arousal
(4) and in modulating amygdala responses
(3,
34), and decreased medial prefrontal cortex activity has been reported in PTSD patients
(3) . In rats, the medial prefrontal cortex homologues modulate hypothalamic-pituitary-adrenal axis responses to psychogenic stressors, provide inhibitory inputs to the PVN
(5), and are involved in extinction recall
(6) . If the dorsal medial prefrontal cortex is indeed involved in inhibitory modulation of integrated response to threat (including hypothalamic-pituitary-adrenal axis activation) and these “modulatory” regions act in concert with the insula and rostral anterior cingulate cortex, the covariation of adrenocorticotropic hormone responses with the dorsal medial prefrontal cortex may reflect this compensatory/modulation role of the dorsal medial prefrontal cortex. Thus, initial activation in modulatory circuits could correlate positively with the magnitude of acute adrenocorticotropic hormone response, while the inhibitory effects might be seen in a shortened duration of response. Follow-up studies with a longer time course of adrenocorticotropic hormone monitoring are needed to test this hypothesis. If our interpretation is correct, this (to the best of our knowledge) is the most direct evidence to date of dorsal medial prefrontal cortex regulation of the hypothalamic-pituitary-adrenal axis in humans, which is consistent with medial prefrontal cortex regulation of the hypothalamic-pituitary-adrenal axis in animals
(5) . In this scenario, hypothalamic-pituitary-adrenal hyper-reactivity may be a result of a failure to recruit medial prefrontal cortex modulatory circuits in concert with hypothalamic-pituitary-adrenal activating regions, which, in the present study, is reflected in the apparent lack of functional coupling of adrenocorticotropic hormone responses with dorsal medial prefrontal cortex activity in combat-PTSD patients.
Cortisol Modulation of Cortical Regions
The data presented in the present study also suggest that circulating cortisol levels modulate paralimbic activity (
Figure 2 ). Voxel-wise analysis of prescan cortisol levels revealed covariation with subsequent rCBF responses in specific paralimbic regions. Effects of cortisol on brain and emotional processing have traditionally been conceptualized in terms of genomic effects of chronically elevated glucocorticoids. However, stress-level glucocorticoids also have rapid nongenomic effects (within minutes) on mammalian neurons, including effects on membrane excitability
(35), which provided a mechanism for rapid, dynamic modulation of brain activity by cortisol. Our data suggest that circulating cortisol may modulate affective/cognitive processing of subsequent stimuli by modulating limbic and/or paralimbic activity in humans.
The covariation of cortisol with activity in the rostral anterior cingulate cortex was found in all of the study groups, whereas cortisol covariation with the subgenual anterior cingulate cortex was observed only in combat-PTSD patients. If the rostral anterior cingulate cortex is directly involved in hypothalamic-pituitary-adrenal axis activation, as suggested in this study, it is not surprising that it might also be responsive to circulating cortisol levels. However, the rostral anterior cingulate cortex has also been implicated in other psychological functions (self-relevance, social emotions)
(36) . It is possible that circulating cortisol modulates rostral anterior cingulate cortex activity subserving these processes. This is consistent with evidence suggesting that the hypothalamic-pituitary-adrenal axis is particularly sensitive to social threat
(11) and with our finding of cortisol covariation with the superior temporal gyrus, which has been implicated in social aspects of emotion
(37) . Activity in the subgenual anterior cingulate cortex has been associated with the induction of sadness
(4,
38), and reduced metabolism in this region has been observed with remission of major depression
(4) . Our finding that prestimulus cortisol covaried with subgenual anterior cingulate cortex activity only in combat-PTSD patients may suggest a PTSD-specific sensitivity to cortisol in regions linked to mood regulation. The combat-PTSD group also showed covariation between cortisol and rCBF in the right temporal polar cortex and bilateral superior temporal cortex. The temporal poles have also been implicated in the processing of aversive emotional stimuli and anticipatory and lactate-induced anxiety. The temporal poles receive auditory inputs from the superior temporal cortex and are reciprocally connected with the medial prefrontal cortex in macaque
(39), which suggests that these temporal cortical regions may participate in circuits that communicate emotional auditory information to the medial frontal network. Although speculative, our finding of cortisol-rCBF covariation in the superior temporal cortex, temporal pole, and subgenual anterior cingulate cortex in combat-PTSD patients suggests that cortisol may potentially “prime” the circuits that detect emotional cues in auditory stimuli.
Interestingly, we did not find covariations of cortisol with the hippocampus, which is perhaps because of our use of an emotion-induction paradigm. Previous studies have reported negative between-subject correlations of baseline cortisol and resting hippocampal rCBF
(14) and decreased hippocampus activity following cortisol administration during explicit memory tasks
(40) . In contrast, we examined brain regions in which activity varied with endogenous cortisol levels. Furthermore, while cortisol administration may decrease hippocampal rCBF during declarative memory tasks, there is no evidence for similar effects in emotional-induction paradigms. Future work is needed to study the links between cortisol and the hippocampus during emotion processing tasks.
There are several limitations to the present study. The results are covariations, and do not demonstrate causality. However, the temporal linkage of emotional challenge, simultaneous brain activity, and adrenocorticotropic hormone levels obtained 5 minutes later suggests meaningful physiological relationships between rCBF and subsequent adrenocorticotropic hormone levels. Cortisol responses to individual challenges could not be resolved because of the sampling schedule, and thus correlations of brain activity with poststimulus cortisol were not examined. Future studies using administration of exogenous cortisol will be helpful in verifying and expanding the present findings. The absence of adrenocorticotropic hormone covariation with any brain regions in the noncombat-exposed healthy comparison subjects should be interpreted with caution. Personalized scripts in this group recounted sad/stressful but not traumatic/threatening events, and thus lower threat salience may explain smaller emotional and endocrine responses. Although this limited our ability to generalize some of our covariation findings to a healthy, nontrauma exposed population, it also highlights the value of using a sensitized population in this type of study.
In conclusion, our study provides evidence of in vivo involvement of specific cortical regions in dynamic hypothalamic-pituitary-adrenal axis responses to emotional stimuli in humans. Acute adrenocorticotropic hormone responses to autobiographical trauma scripts in combat veterans suggest that the experience of personal threat may sensitize the hypothalamic-pituitary-adrenal axis to subsequent recall. Covariation of plasma adrenocorticotropic hormone with activity in the anterior insula, rostral anterior cingulate cortex, and dorsal medial prefrontal cortex implicates these regions in activation and/or modulation of hypothalamic-pituitary-adrenal axis responses in humans, which is consistent with evidence in animal models. The covariation of cortisol levels with activity in the rostral and subgenual anterior cingulate cortex, which are regions associated with self-induced sadness/depression, suggests that activity in the anterior cingulate cortex may be modulated by circulating cortisol during emotional processing, potentially sensitizing limbic structures toward aversive stimuli.