Fear conditioning, the associative learning process by which a neutral conditioned stimulus acquires the capacity to elicit fear following its repeated pairing with an aversive unconditioned stimulus, features prominently in etiologic accounts of social anxiety disorder
(1 –
3) . In this context, the unconditioned stimulus consists of a time-limited humiliating experience and the conditioned stimuli are those stimuli associatively linked to the unconditioned stimulus (i.e., people, places, and things)
(1) . Conditioning is thought to contribute to the onset and course of social anxiety disorder by conferring anxiogenic valence to conditioned stimuli that are then capable of sustaining social anxiety beyond the presence of the time-limited unconditioned stimulus.
Theories regarding conditioning and social anxiety disorder and other anxiety disorders are not without controversy
(4) . Empirical evidence for a conditioning contribution to social anxiety disorder stems largely from studies documenting high frequencies of retrospectively reported traumatic conditioning as a precipitant of the disorder
(2,
5,
6) . However, the strength of such retrospective and subjective evidence is compromised by the often inaccurate recall of events occurring many years earlier
(7), the undue influence of leading questions regarding the origins of the phobia
(8), and evidence that fear conditioning processes can occur on an unconscious level, inaccessible to subjective experience
(9,
10) . Furthermore, many healthy individuals recall embarrassing conditioning experiences but do not go on to develop the disorder
(6), indicating that adverse social conditioning encounters are not a sufficient circumstance for the development of social anxiety disorder.
Psychophysiological paradigms in which social anxiety patients and healthy comparison subjects are classically conditioned in a laboratory setting are an alternative and promising means to further assessing the conditioning contribution to social anxiety disorder. Specifically, such paradigms do not rely on retrospective or subjective data and are capable of assessing individual differences in conditioning that may help explain why some exposed to conditioning precipitants but not others go on to develop the disorder.
To date, three such studies have been conducted in social anxiety patients
(11 –
13) . These studies assessed the degree to which those with social anxiety disorder display a general proclivity toward forming aversive associations. Because of this focus on nonspecific conditionability, these studies conditioned subjects using generally aversive but socially irrelevant unconditioned stimuli such as unpleasant odors
(11,
12) and painful pressure
(13) . Results demonstrated no increase in conditioning among social anxiety subjects, whether the observed conditioned response was fear-potentiated startle
(11), skin conductance
(11,
13), or heart rate
(12) . These results suggest the absence of a general proclivity toward forming aversive associations among subjects with social anxiety disorder. Nevertheless, the involvement of a more specific proclivity among social anxiety patients to form aversive associations between socially relevant unconditioned stimuli (e.g., a humiliating experience) and co-occurring neutral conditioned stimuli (i.e., people, places, and things) remains to be tested.
In the present study, subjects with social anxiety and gender- and age-matched healthy comparison subjects underwent differential fear conditioning within a novel paradigm incorporating socially stressful unconditioned stimuli with demonstrated relevance to social anxiety disorder, such as critical facial expressions
(14,
15) and derogatory verbal feedback
(16,
17) . Neutral facial expressions served as conditioned stimuli and were repeatedly paired with the aversive unconditioned stimulus. The conditioned response was measured as fear-potentiated startle elicited by presentation of the conditioned stimuli. Fear-potentiated startle refers to the reliable enhancement of the startle reflex when an organism is in a state of fear
(18) and is employed as an objective measurement of anxious arousal in humans
(19) . The central hypothesis of our study predicted greater conditioned fear-potentiated startle in subjects with social anxiety disorder than subjects without the disorder. To our knowledge, the present study represents the first psychophysiological test of conditioning correlates of social anxiety within a paradigm employing unconditioned stimuli specifically relevant to the diagnosis of social anxiety disorder.
Method
Participants
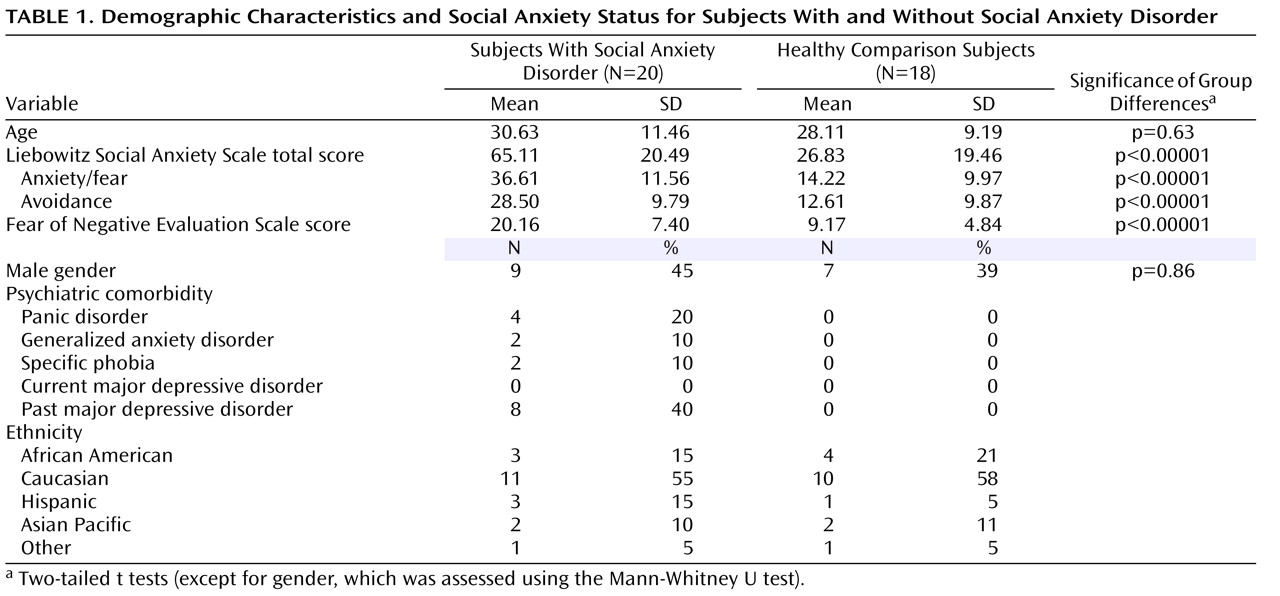
Subjects with a DSM-IV-TR diagnosis of generalized social anxiety disorder (N=20) and age- and gender-matched healthy comparison subjects (N=18) served as study participants. Demographic characteristics as well as psychiatric comorbidities for both subject groups are provided in
Table 1 . All subjects with social anxiety disorder met criteria for a current diagnosis of generalized social anxiety disorder as determined by the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P)
(20), which was administered by one of four staff psychologists (interrater reliability: kappa=0.76). Additionally, these subjects were independently assessed by a senior psychiatrist (D.S.P.) to confirm the SCID-I/P diagnoses. Finally, the self-report version of the Liebowitz Social Anxiety Scale
(21) and the Fear of Negative Evaluation Scale
(22) were completed by all participants to provide a continuous measure of social anxiety traits.
Comparison subjects were required to be free of current or past axis I psychopathology as per the Structured Clinical Interview for DSM-IV (SCID). Diagnostic exclusion criteria for social anxiety subjects included 1) current major depressive disorder or suicidal ideation, 2) history of alcohol or substance abuse or dependence (other than nicotine) within 6 months of study start, and 3) current or past history of bipolar depression, psychosis, or delusional disorders. Additionally, exclusion criteria for all participants included 1) use of psychopharmacologic medication or other drugs capable of altering central nervous system function within 2 weeks of testing or use of fluoxetine within 6 weeks of testing, 2) current use of illicit drugs (as defined by SCID and confirmed with a urine test), 3) pregnancy, and 4) medical conditions or treatment for conditions that interfered with the objectives of the study as determined by a staff physician during a physical exam. Experimental procedures were described in detail and participants gave written informed consent, which was approved by the National Institute of Mental Health’s Human Investigation Review Board.
Procedure
Stimulation and recording were controlled by a commercial device (PsyLab SAM System Contact Precision Instruments, London). Electromyography (EMG) of the startle reflex (eye blink) was recorded using two 6-mm tin cup electrodes placed under the right eye. Amplifier bandwidth was set to 30–500 Hz and digital data were sampled at 1000 Hz. The startle eye blink was elicited by a 40-msec, 3-psi puff of compressed air delivered to the center of the forehead through a polyethylene tube (2.0 ft long, 1/8 inch inside diameter) affixed 1 centimeter from the skin by way of a headpiece worn by the subject. A visor was positioned between the polyethylene tube and the subject’s eyes to prevent the puff of air from reaching the cornea. This probe setup was identical to that shown to work effectively in previous studies
(23,
24) .
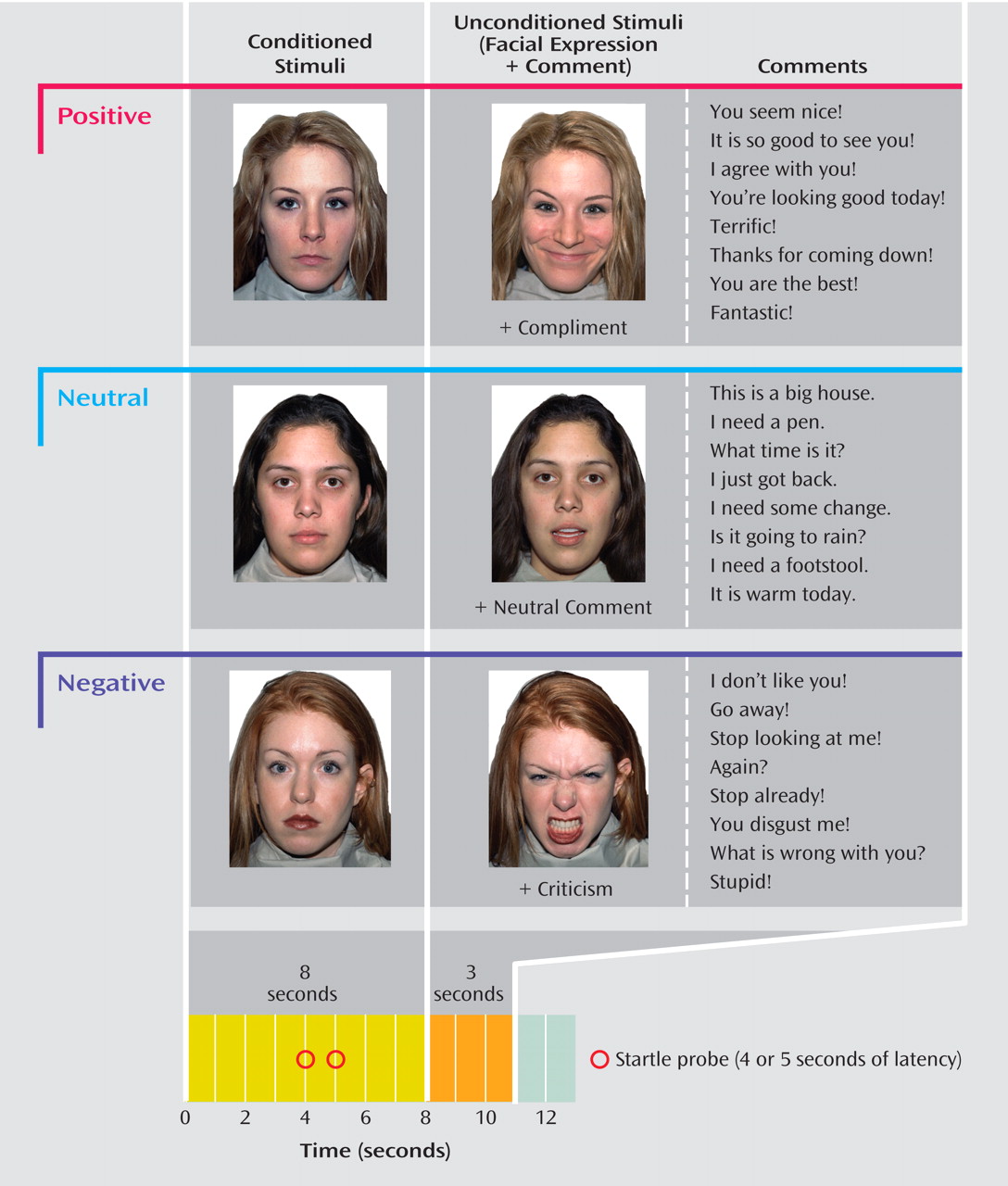
We employed a differential fear conditioning paradigm, during which visual conditioned stimuli (duration=8 sec) consisting of neutral facial expressions from three female actors (blonde, brunette, and redhead) were each paired with one of three classes of audiovisual unconditioned stimuli (85 dB, duration=3 sec): 1) insults and critical facial expressions (US
neg ), 2) neutral comments and neutral facial expressions (US
neu ), and 3) compliments and positive facial expressions (US
pos ). Conditioned stimuli paired with negative, neutral, and positive unconditioned stimuli were referred to as CS
neg, CS
neu, and CS
pos, respectively. The preconditioning sequence comprised of 12 trials; each trial included four presentations of each type of conditioned stimulus without any unconditioned stimuli. The conditioning sequence comprised of 24 trials; each trial included eight presentations of each type of conditioned stimulus paired with its respective unconditioned stimulus. Onset of the unconditioned stimulus (facial expression plus comment) was concurrent with cessation of the conditioned stimulus (i.e., 8 seconds poststimulus). The extinction sequence comprised of 24 trials and was similar to that of the preconditioning sequence (unconditioned stimuli were absent). The conditioned stimuli during each of the three sequences were quasi-randomly ordered so that no more than two conditioned stimuli of the same class were consecutively presented. Additionally, the assignment of actors to type of conditioned stimulus (negative, neutral, or positive) was counterbalanced across subjects so that each actor (blonde, brunette, and redhead) served as CS
neg, CS
neu, or CS
pos for an equal number of subjects.
Figure 1 displays a schematic summary of the paradigm.
At the outset of the study, nine startle probes were delivered to habituate the startle reflex prior to data collection (interprobe interval=18–25 sec). In order to elicit startle responses during CS neg, CS neu, and CS pos presentations at preconditioning, conditioning, and extinction, startle probes were delivered 4 to 5 seconds following each conditioned stimulus and an interprobe interval of 18 to 25 seconds was maintained throughout. Preconditioning directly preceded conditioning. Immediately following conditioning and extinction, subjects rated their reactions to the conditioned stimuli using a questionnaire in which each type of conditioned stimulus was rated on a 10-point Likert scale reflecting the level of anxiety, unpleasantness, and arousal (1=none, 10=extreme levels). Additionally, following conditioning but before extinction, each type of unconditioned stimulus was rated on a 10-point scale reflecting the level of happiness and anxiety elicited by each of the three unconditioned stimuli.
Data Analysis
EMG of the startle reflex was rectified and smoothed by using moving averages with a 20-msec window. The window for onset latency for the blink reflex was 20 to 100 msec, and the peak magnitude was determined within 120 msec after response onset. Additionally, the EMG level for the 50 msec preceding the startle probe was subtracted from the peak magnitude. EMG magnitudes were standardized using within-subject paired t tests. In order to capture the learning curves for startle modulation, conditioning and extinction sequences were each divided into four blocks, each including two conditioned stimuli of each type. Startle data were analyzed with 2-by-3-by-4-by-2 multivariate analysis of variance (MANOVA) with repeated measures. Subject group (social anxiety, healthy comparison), type of conditioned stimulus (negative, neutral, positive), time (block 1, 2, 3, 4), and gender were within-subject factors. Subjective reactions to conditioned stimuli (anxiety, unpleasantness, or arousal) were analyzed with 2-by-3-by-2 MANOVA with repeated measures; subject group, type of conditioned stimulus, and gender were within-subject factors. An alpha level of 0.05 was accepted as a nominal level of significance. Because the main and interaction effects of gender were not significant for both EMG and self-report data, gender results are not presented below.
Results
Startle EMG
Preconditioning
The main effect of type of conditioned stimulus was nonsignificant (p=0.51). Additionally, both the type of conditioned stimulus by subject group and type of conditioned stimulus by time interactions were nonsignificant (both p values >0.26).
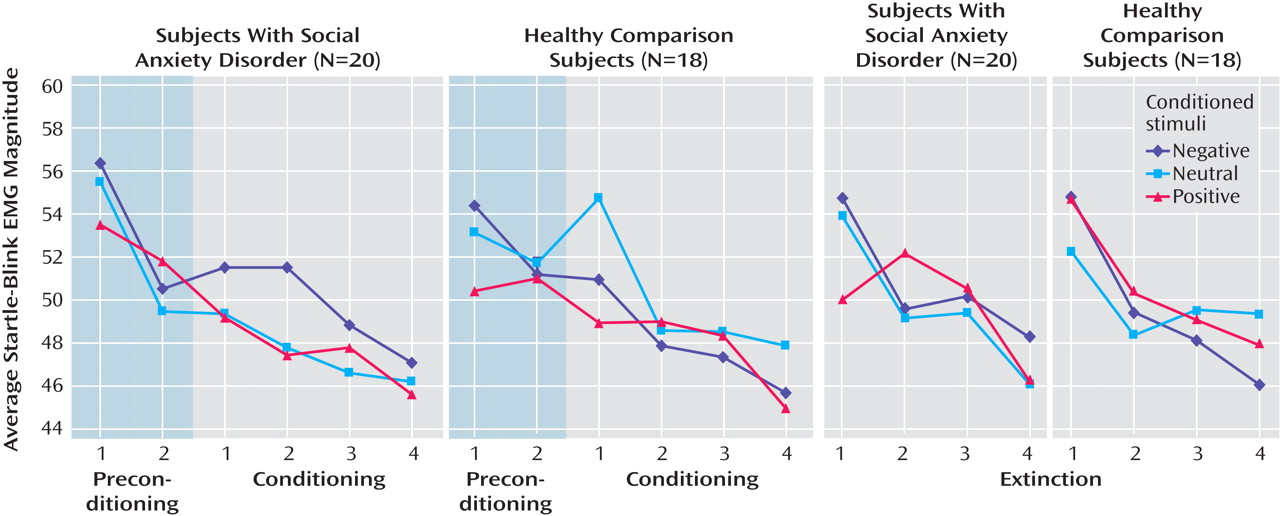
Conditioning
Though the main effect of type of conditioned stimulus and the type of conditioned stimulus by time interaction were both nonsignificant (both p values >0.13), a type of conditioned stimulus by subject group interaction emerged (F=3.79, df=2, 34, p=0.03), indicating different patterns of response to conditioned stimuli across subject groups during conditioning (
Figure 2 ). Analyses of simple effects of type of conditioned stimulus calculated separately for each subject group revealed a significant difference in startle magnitudes across type of conditioned stimulus in social anxiety subjects (F=3.79, df=2, 18, p<0.04) but not healthy subjects (p>0.26). Planned contrasts designed to test differences between types of conditioned stimuli at conditioning for each subject group revealed greater startle EMG for CS
neg versus both CS
neu (t=2.36, df=20, p=0.03) and CS
pos (t= 2.52, df=20, p=0.02) among social anxiety subjects and no effects of type of conditioned stimulus among healthy subjects. These results indicate that social anxiety subjects but not healthy subjects displayed fear-potentiated startle in response to neutral faces paired with critical facial expressions and insults. This difference between subject groups was not likely due to effects of arousal, as CS
neg and CS
pos did not differ on arousal (p=0.20), yet CS
neg relative to CS
pos elicited startle potentiation among social anxiety subjects but not healthy subjects.
To further examine the relationship between severity of social anxiety and fear conditioning, continuous Liebowitz Social Anxiety Scale and Fear of Negative Evaluation Scale scores were correlated with the conditioned response of fear-potentiated startle. Results indicated a significant correlation between fear-potentiated startle and Fear of Negative Evaluation Scale score (r=0.33, df=39, p=0.04) and a correlation between fear-potentiated startle and Liebowitz Social Anxiety Scale total score (r=0.30, df=39, p=0.08). The direction of these correlations suggests greater conditioned response among those subjects experiencing higher levels of social anxiety.
Extinction
The main effect of type of conditioned stimulus, as well as subject group by type of conditioned stimulus and type of conditioned stimulus by time interactions, was nonsignificant (all p values >0.48). Additionally, type of conditioned stimulus effects and type of conditioned stimulus by time interaction effects analyzed separately for each subject group were nonsignificant (all p values >0.50). Such results suggest that conditioned response was relatively absent during the extinction sequence for social anxiety subjects and healthy subjects alike (
Figure 2 ).
Self-Reported Reactions to the Conditioned Stimulus
Preconditioning
The main effect of type of conditioned stimulus and the type of conditioned stimulus by subject group interaction were nonsignificant for all self-report measures (all p values >0.27).
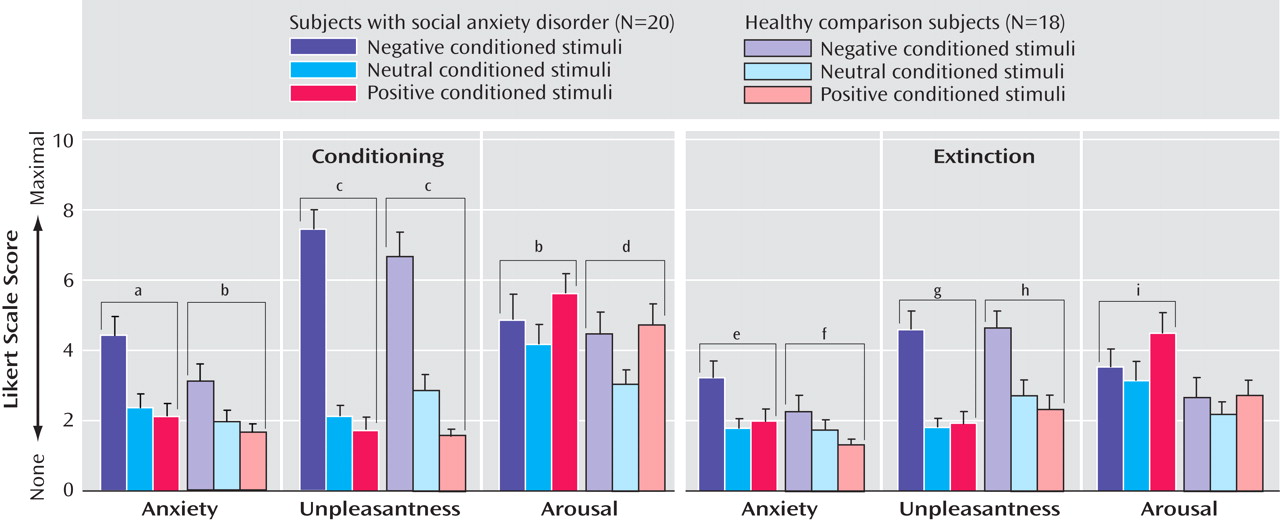
Conditioning
Average levels of self-reported anxiety, unpleasantness, and arousal across subject groups are displayed in
Figure 3 . Main effects of type of conditioned stimulus were found for self-reported measures of anxiety (p<0.0001), unpleasantness (p<0.0001), and arousal (p=0.001), with linear increases from CS
pos to CS
neu to CS
neg for anxiety and unpleasantness (both p values <0.0005) and a quadratic trend for arousal (p=0.002), due to an inverted U-shaped distribution from CS
neg to CS
neu to CS
pos and no difference in arousal between CS
neg and CS
pos (p=0.20). However, the type of conditioned stimulus by subject group interactions for unpleasantness (p=0.13), anxiety (p=0.45), and arousal ratings (p=0.77) were all nonsignificant.
Extinction
Similar to conditioning results, main effects of type of conditioned stimulus were found for anxiety (p=0.005), unpleasantness (p<0.0001), and arousal ratings (p=0.013), with linear increases from CS
pos to CS
neu to CS
neg for both anxiety and unpleasantness (both p values <0.008) and arousal levels forming an inverted U-shaped distribution from CS
neg to CS
neu to CS
pos (p=0.02) (
Figure 3 ). Additionally, the type of conditioned stimulus by subject group interaction was nonsignificant for unpleasantness (p=0.34), anxiety (p=0.14), and arousal ratings (p=0.29).
Self-Reported Reactions to the Unconditioned Stimulus
Main effects of type of unconditioned stimulus were found for self-reported measures of happiness and anxiety (all p values <0.001), with mean anxiety ratings increasing linearly from US pos (1.62, SD=0.99) to US neu (1.87, SD=1.38) to US neg (3.97, SD=2.50) and mean happiness ratings decreasing linearly from US pos (6.74, SD=1.39) to US neu (4.72, SD=1.17) to US neg (3.23, SD=1.58). Subject group did not interact with type of unconditioned stimulus for anxiety or happiness ratings (all p values >0.21), and US neg received higher anxiety ratings and lower happiness ratings than either US neu or US pos among both social anxiety subjects (all p values <0.001) and healthy subjects (all p values <0.01). Notably, mean increases in reported anxiety from US neu to US neg among social anxiety subjects (4.55, SD=2.61) were greater than those of the healthy subjects (3.37, SD=2.29), though the within-group difference was nonsignificant (p=0.14).
Social Anxiety Traits and Conditioned Fear-Potentiated Startle
The nonsignificant (p=0.14) finding that anxiety ratings for the unconditioned stimulus were greater among social anxiety subjects complicates interpretation of the conditioned response, in that greater conditioned fear-potentiated startle among these subjects may be a function of either heightened proclivity toward forming aversive associations (hyperconditionability) or increased unconditioned stimulus reactivity resulting in increased conditioned response
(25 –
27) . As a result, startle reflex data were reanalyzed using multiple linear regression to assess the combined and unique contributions of unconditioned stimulus reactivity and social anxiety status to levels of conditioning at acquisition. The level of conditioned startle-potentiation (CS
neg –CS
neu ) was entered as the dependent measure and both social anxiety status and unconditioned stimulus reactivity were simultaneously entered as predictors. Separate analyses were conducted with social anxiety status represented by either diagnosis (social anxiety versus healthy), Fear of Negative Evaluation Scale score, or Liebowitz Social Anxiety Scale score. It is important to note that collinearity levels indicated sufficiently low levels of variance inflation for both predictors (all levels <1.31), whether analyzing social anxiety by diagnostic status, Fear of Negative Evaluation Scale score, or Liebowitz Social Anxiety Scale score. This indicates sufficiently low collinearity for simultaneous entry of social anxiety status and unconditioned stimulus reactivity in regression analyses
(28) .
Together, diagnostic status and unconditioned stimulus reactivity accounted for 22% of the variance in levels of acquisition (F=4.95, p=0.013), with diagnostic status contributing 18% of the variance (b=0.431, p=0.008) and unconditioned stimulus reactivity making a nonsignificant contribution (b=0.114, p=0.46). Regression results from the analysis including Fear of Negative Evaluation Scale score rather than diagnostic status produced similar results, with a significant combined contribution of Fear of Negative Evaluation Scale score and unconditioned stimulus reactivity to the variance (R 2 =0.16, F=3.30, df=2, 34, p<0.05); Fear of Negative Evaluation Scale score (b=0.40, p=0.03), but not unconditioned stimulus reactivity (b=0.014, p=0.94), uniquely contributed to levels of variance at acquisition. Finally, the regression analysis including Liebowitz Social Anxiety Scale score yielded comparable results, with a combined contribution from Liebowitz Social Anxiety Scale score and unconditioned stimulus reactivity (R 2 =0.14, p=0.09) and a unique contribution from Liebowitz Social Anxiety Scale score (b=0.31, p=0.08) but not unconditioned stimulus reactivity (b=0.15, p=0.39). These results demonstrate that the positive association between social anxiety traits and conditioned fear-potentiated startle is not driven by levels of unconditioned stimulus reactivity, as social anxiety status and Fear of Negative Evaluation Scale and Liebowitz Social Anxiety Scale scores are significant predictors of conditioning even after the variance associated with unconditioned stimulus reactivity is statistically removed.
Discussion
A novel social conditioning paradigm employing the socially relevant unconditioned stimuli of critical facial and verbal feedback produced evidence of conditioning abnormalities among subjects with social anxiety disorder. Specifically, the present study found elevated levels of conditioned fear-potentiated startle in subjects with social anxiety disorder relative to healthy comparison subjects. Additionally, both fear-potentiated startle and self-reported reactions of unpleasantness in response to the negative conditioned stimulus increased as scores on the Liebowitz Social Anxiety Scale increased, demonstrating stronger conditioned responses with increasing levels of social anxiety disorder symptoms. Furthermore, subject group differences in conditioning likely resulted from the social anxiety valence of the CS neg rather than nonspecific arousal generated by the CS neg, as differences in startle potentiation between subjects groups were not restricted to CS neg versus CS neu comparisons but also emerged during the arousal-controlled comparison of CS neg versus CS pos . It is important to note that greater conditioned response among subjects with social anxiety, as well as the positive relationship between continuous social anxiety traits and fear conditioning, was not driven by unconditioned stimulus reactivity, as such effects remained after statistically controlling for levels of anxiety elicited by the unconditioned stimulus.
Social Anxiety Disorder and Selective Sensitivity to Social Conditioning
Current findings reflecting subject-comparison differences in levels of conditioning to socially relevant unconditioned stimuli contrast with the null subject-comparison differences in levels of conditioning to socially irrelevant unconditioned stimuli (e.g., unpleasant odors and painful pressure) found in past psychophysiological studies of social anxiety disorder
(11 –
13) . These results suggest that social anxiety disorder includes a selective sensitivity to social anxiety-related conditioning experiences, rather than a general proclivity toward associative fear conditioning. One candidate mechanism for this selective sensitivity derives from findings of pronounced stress-related increases in glucocorticoids and epinephrine among social anxiety patients
(29,
30) . Because such stress hormones have been shown to enhance the stability and longevity of human memory through consolidation
(31,
32), hyper-release of stress hormones among social anxiety subjects during social conditioning may result in greater levels of conditioned fear response via memory consolidation. Conversely, socially irrelevant conditioning experiences that would not be thought to lead to greater release of stress hormones among social anxiety subjects would be predicted to generate similar levels of conditioned fear across those with and without social anxiety disorder. This proposed neuroendocrinological path by which social anxiety subjects display increased social conditioning may represent an important component of social anxiety disorder pathophysiology and awaits formal empirical testing.
Startle EMG and Self-Reported Measures of Conditioning
Whereas EMG results significantly demonstrated increases in conditioned response among subjects with social anxiety disorder versus healthy comparison subjects, subject group differences in self-reported measures fell below significance. An additional difference between self-reported and psychophysiological results was that comparison subjects showed evidence of learning by the former but not latter measure of conditioning. Because effects of conditioned startle-potentiation have been shown to require a more highly aversive unconditioned stimulus than other measures of conditioning
(33,
34), it is plausible that the US
neg was experienced aversively enough by comparison subjects to elicit conditioning (as measured via self-report) but was insufficiently aversive to generate conditioned startle-potentiation.
Strengths and Limitations
The strength of this study is the introduction of a novel, ecologically enhanced social conditioning paradigm capable of eliciting fear conditioning abnormalities associated with social anxiety disorder in the laboratory environment. Future applications of this paradigm in neuroimaging and pharmacological research may offer pathophysiological insights critical to an eventual brain-based taxonomy for social anxiety disorder, as well as novel targets for pharmacological intervention. However, before moving to this next phase of research, the limitations of the current study should be addressed.
The primary limitation of this study derives from the relative absence of conditioned startle-potentiation among either social anxiety subjects or comparison subjects during the extinction sequence. Though this type of fast extinction of conditioned startle-potentiation is not atypical
(35), it nevertheless compromises the paradigm’s capacity for measuring extinction, a clinically important inhibitory learning process shown to be delayed in anxiety patients
(36,
37) . A closer look at conditioning data reveals a weakening of the conditioned response over the course of acquisition (
Figure 2 ) suggestive of habituation to the unconditioned stimulus during acquisition training. Such habituation toward the end of acquisition likely resulted in significantly reduced anxious anticipation of the unconditioned stimulus, emotion required for the conditioned response to last into the extinction sequence. Future applications of this paradigm might prevent undue habituation to the unconditioned stimulus by reducing the reinforcement rate from 100% (i.e., eight unconditioned stimuli of each type) to 50% (i.e., four unconditioned stimuli of each type). Additionally, future studies should include objective measures of reaction to the unconditioned stimulus to track habituation and to provide further testing of the contribution made by unconditioned stimulus reactivity to differential levels of conditioning across subject groups.
An additional limitation of this study is the lack of information clarifying whether the greater conditioning response found in social anxiety subjects is an etiological precursor of the disorder or an epiphenomenon of the active disease process. Studies using longitudinal designs to collect pre- and postmorbid conditioning levels among social anxiety subjects are needed. The high-risk paradigm may be best suited for such a task
(38,
39), whereby healthy individuals with the greatest likelihood of developing clinical anxiety (e.g., biological offspring of adults with social anxiety disorder
[40] ) would be tracked over time. Premorbid conditioning processes could then be compared across individuals who do and do not develop social anxiety to identify conditioning precipitants of the disorder.
Conclusion
The current study introduces a laboratory-based social conditioning paradigm incorporating the socially relevant unconditioned stimuli of critical facial expressions and verbal feedback. In contrast to null results of past studies employing socially irrelevant unconditioned stimuli, the current findings demonstrate facilitated conditioning among subjects with social anxiety disorder. Such results highlight the importance of using disorder-specific unconditioned stimuli and validate the current paradigm as a novel experimental means to studying the neurobiology, pharmacology, and genetics of conditioning abnormalities associated with social anxiety disorder.