The serotonin, or 5-HT (5-hydroxytryptamine), system is critically involved in the pathophysiology of mood and anxiety disorders, and drugs that target serotonergic neurotransmission are efficacious for the treatment of these disorders
(1) . The serotonin transporter (5-HTT) plays an important role in serotonergic neurotransmission by facilitating reuptake of 5-HT from the synaptic cleft. A repeat of 20–23 base pairs has been observed as a motif within a polymorphic region of the serotonin transporter gene, and it occurs as two prevalent alleles: one consisting of 14 repeats (the short allele variant) and another of 16 repeats (the long allele variant). This functional polymorphism in the 5- regulatory promoter region, termed 5-HTTLPR (5-HTT-gene-linked polymorphic region), alters transcription of the serotonin transporter gene
(2) . Specifically, the short allele of the 5-HTTLPR polymorphism is thought to reduce transcription efficiency for the gene, resulting in decreased expression of the serotonin transporter and decreased serotonin uptake in lymphoblast cell lines and blood platelets
(3,
4) and decreased availability of the serotonin transporter in human imaging studies
(5) . The s allele appears to exert its effect independent of s allele load, i.e., in a dominant manner
(6) .
With remarkable consistency (exceptions are studies by Surtees et al.
[7] and Gillespie et al.
[8] ), the s allele has been shown to interact with stressful life events to predict depression (9–16) in medically healthy subjects. In addition, 5-HTTLPR may predispose to depression in patients with severe medical illnesses that are associated with ongoing chronic stress
(17) . Thus, in study groups in which all patients are chronically ill, resulting in a considerable amount of stress, one might expect to find a direct effect of 5-HTTLPR on depression. Indeed, in several small studies the s allele of 5-HTTLPR has been associated with depression in patients with Parkinson’s disease
(18,
19), in those with stroke
(20), and in elderly patients after hip fracture
(21) . One Japanese study suggested that the s allele may be associated with depression in patients after acute coronary syndrome
(22) .
Whether these results are applicable to patients with stable coronary disease, particularly given substantial differences in allele frequency in 5-HTTLPR across populations, is unknown. We performed a cross-sectional study to determine whether the s allele of 5-HTTLPR is associated with current depression, greater perceived stress, and higher 24-hour urinary norepinephrine excretion among a group of 557 white patients with stable coronary disease who were enrolled in the Heart and Soul Study.
Method
Participants
Details regarding our recruitment procedures have been published previously
(23 –
25) . In brief, we used administrative databases to identify outpatients with documented coronary disease at two Department of Veterans Affairs (VA) medical centers in California (San Francisco VA Medical Center and VA Palo Alto Health Care System), one university medical center (University of California, San Francisco), and nine public health clinics in the Community Health Network of San Francisco. Patients were eligible to participate if they had at least one of the following: a history of myocardial infarction, angiographic evidence of ≥50% stenosis in one or more coronary blood vessels, prior evidence of exercise-induced ischemia by treadmill or nuclear testing, or a history of coronary revascularization. Between September 2000 and December 2002, a total of 1,024 participants enrolled and completed a daylong study appointment at the San Francisco VA Medical Center. In genetic association studies, ethnically homogenous study groups are recommended because of the problem of population stratification
(26,
27) . To avoid population stratification, we chose to examine genotypes only in white patients, the largest group in the Heart and Soul Study (N=557). Our protocol was approved by the appropriate institutional review boards. After complete description of the study to the subjects, written informed consent was obtained.
Assessment of Depression
We assessed current (past month) depression by using the Computerized National Institute of Mental Health Diagnostic Interview Schedule (CDIS-IV)
(28), a highly structured interview designed to yield psychiatric diagnoses according to DSM-IV criteria. The Diagnostic Interview Schedule (DIS) has been used extensively to study the epidemiology and treatment of depression
(29) . Trained research assistants (who were blinded to 5-HTTLPR status) administered the interview during the daylong baseline study appointment. Participants found to have current depression were informed that they were suffering from depression, instructed to discuss these symptoms with their primary care provider, and provided a list of local resources available for further evaluation and treatment.
Assessment of Perceived Stress
We measured perceived stress by using a four-item version of the Perceived Stress Scale
(30), a brief and easy-to-administer measure of the degree to which situations in one’s life are appraised as stressful. The scale contains the following questions: 1) “In the last month, how often have you felt that you were unable to control the important things in your life?” 2) “In the last month, how often have you felt confident about your ability to handle your personal problems?” (reverse scored), 3) “In the last month, how often have you felt that things were going your way?” (reverse scored), and 4) “In the last month, how often have you felt difficulties were piling up so high that you could not overcome them?” The responses were scored as 0 (never), 1 (almost never), 2 (sometimes), 3 (fairly often), or 4 (very often). The total score ranges from 0 to 16, with higher scores indicating greater stress. As a dichotomous indicator of perceived stress, we chose a cut point of ≥5 to represent a median split of the study group.
24-Hour Urinary Norepinephrine Excretion
Details regarding the analysis of 24-hour urinary norepinephrine excretion have been described elsewhere in greater detail
(23) . In brief, the participants were instructed to collect all urine for 24 hours between the study appointment and the time when a researcher visited their home the next day and to keep the urine collection jugs refrigerated at all times. Research personnel arrived at the patients’ homes exactly 24 hours after the appointment to ensure accurately timed specimens and to enhance compliance with the protocol. All patients were asked whether they were able to collect all of the urine or if some fraction had been inadvertently discarded. The participants were also asked if the urine had been refrigerated throughout the 24-hour period. If the urine sample was reported to be incomplete or not refrigerated throughout the procedure, or if the volume was less than 1 liter, the subject was asked to repeat the collection, and research personnel returned 24 hours later to recollect the urine. Similarly, if the 3-liter collection jug was completely full, the subject was given two new jugs and asked to repeat the collection to ensure that no urine was inadvertently discarded.
Urinary norepinephrine was measured with gas chromatography–mass spectrometry (by personnel blinded to 5-HTTLPR status) at ARUP Laboratories (headquarters, Salt Lake City). The normal reference range is 0–100 mg/day for norepinephrine. Since the detection limit was 1.0 mg/dl for norepinephrine, levels for participants whose excretion was below this detection limit were coded as 1.0 mg/dl. The interassay coefficient of variance was <10%, and the intra-assay coefficient of variance was <8%.
To ensure adequate sampling for the norepinephrine analysis, we included only participants who had a urinary creatinine value within the normal range (0.8–2.1 g/day). For the norepinephrine analysis, we further excluded participants with medical diseases known to affect measurement of 24-hour urinary norepinephrine, including one participant with a pheochromocytoma, seven participants with incomplete urine collections, and 24 participants whose urine refrigeration was not verified.
Genotyping of 5-HTTLPR
5-HTTLPR genotyping was performed at the Genomics Core Facility at the University of California, San Francisco. The polymorphic promoter region (5-HTTLPR) was amplified with polymerase chain reaction (PCR) using 5′ FAM labeled forward (FAM-GGCGTTGCCGCTCTGAATG) and unlabeled reverse (GAGGGACTGAGCTGGACAACCAC) primers. Amplification was performed in a final volume of 6 ml containing 20 ng genomic DNA template, 200 mmol/liter deoxynucleotide triphosphates, 50 mM Tris, 0.15 mM (NH 4 ) 2 SO 4 (pH adjusted to 9.3 with NH 4 OH), 2.5 mM MgCl 2, 0.1% Tween 20, and 0.25 U JumpStart AccuTaq LA DNA polymerase (Sigma-Aldrich, St. Louis). This was then cycled at 94°C for 1 minute and then underwent 45 cycles at 94°C for 30 seconds and 68°C for 4 minutes, with a final 30 minutes at 68°C. The PCR product was diluted (1:12), combined with 0.35 ml size standard and 9.0 ml highly deionized formamide denatured by heating at 95°C for 5 minutes and analyzed by using a 3730xl DNA analyzer with GeneMapper 4.0 analysis software (Applied Biosystems). The PCR fragment sizes for the long and short alleles are 529 base pairs (16 repeats) and 486 base pairs (14 repeats), respectively.
Potential Confounding or Moderating Variables
Self-reported date of birth, gender, medical history, smoking, and alcohol use were determined by questionnaire. We measured resting blood pressure by using a standard sphygmomanometer and performed a symptom-limited, graded exercise treadmill test according to a standard Bruce protocol. We defined exercise capacity as the total number of metabolic equivalents achieved at peak exercise.
Statistical Analysis
The goal of this investigation was to examine the association of 5-HTTLPR with depression, perceived stress, and norepinephrine secretion in patients with coronary disease. After preliminary analyses confirmed dominance of the s allele, we pursued the remaining analyses with the patients divided according to the s allele (s/s or s/l versus l/l). Differences in characteristics between participants with and without the s allele were determined by using t tests (or nonparametric equivalent) for continuous variables and chi-square tests for dichotomous variables. Analysis of covariance was used to compare mean norepinephrine excretion and mean perceived stress in participants with and without the s allele.
To determine the unadjusted and adjusted associations between s allele status and depression, perceived stress, and norepinephrine, we used logistic regression analyses with s allele status as the predictor variable. Our outcome variables were 1) presence of current depression according to the CDIS, 2) proportion with moderate or high perceived stress (score >5 on Perceived Stress Scale), and 3) proportion in the highest quartile of 24-hour urinary norepinephrine excretion. We also examined the effects of interactions between 5-HTTLPR and potential moderating variables in predicting our outcome variables. We adjusted all analyses for age and gender. The analyses were performed by using Statistical Analysis Software (version 9, SAS Institute, Cary, N.C.).
Results
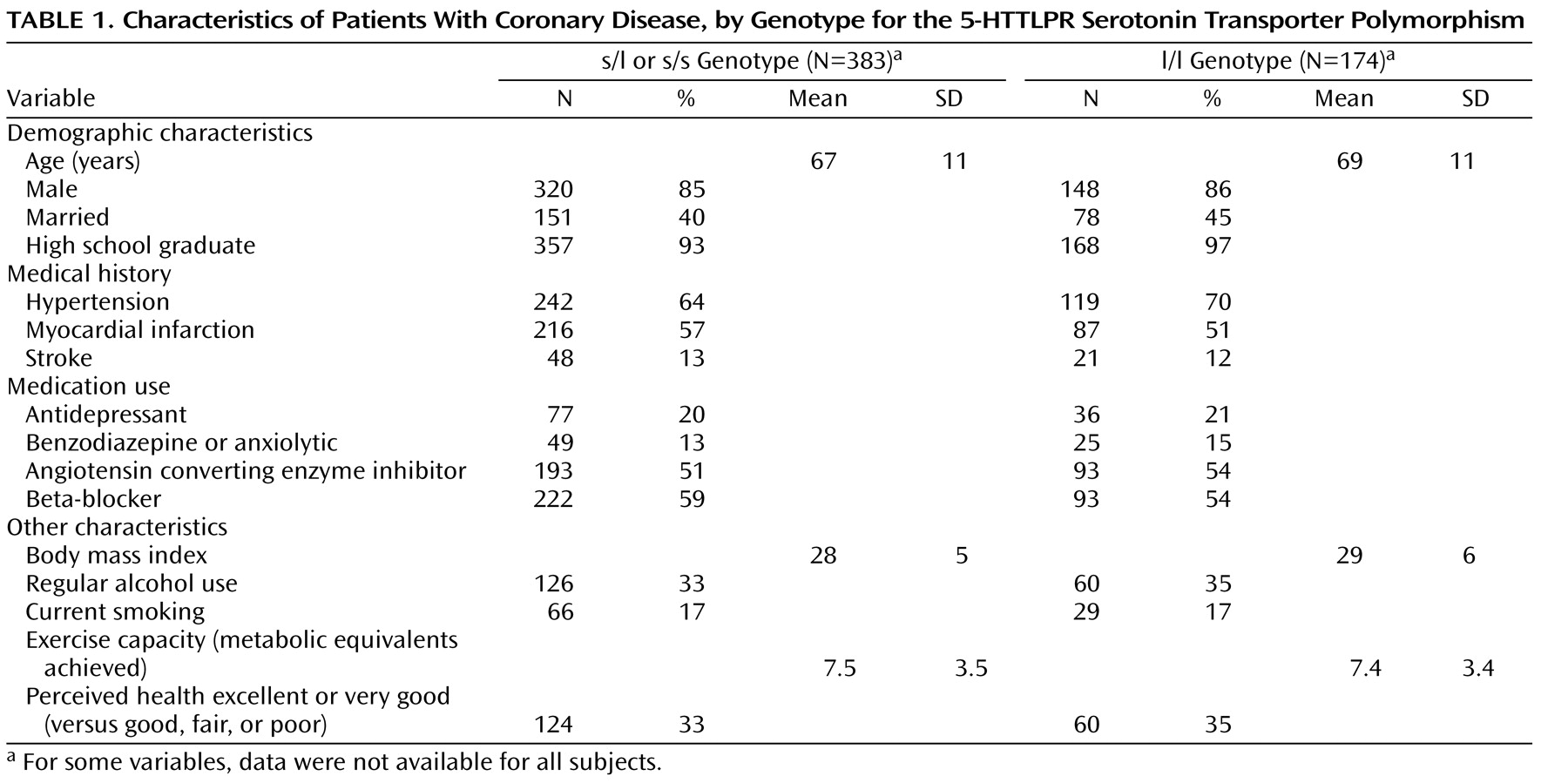
Of the 557 participants, 96 (17%) were homozygous for the s allele (s/s genotype), 287 (52%) were heterozygous (s/l genotype), and 174 (31%) were homozygous for the l allele (l/l genotype). The allele frequencies were almost identical to previously reported frequencies in white populations
(4,
14) . The three groups were in Hardy-Weinberg equilibrium (χ
2 =0.12, df=2, n.s.). No differences in age, sex, medical illness, medication use, body mass index, alcohol use, and cardiac function were observed between s allele carriers and l/l individuals. Characteristics stratified according to s allele status are shown in
Table 1 .
5-HTTLPR and Depression
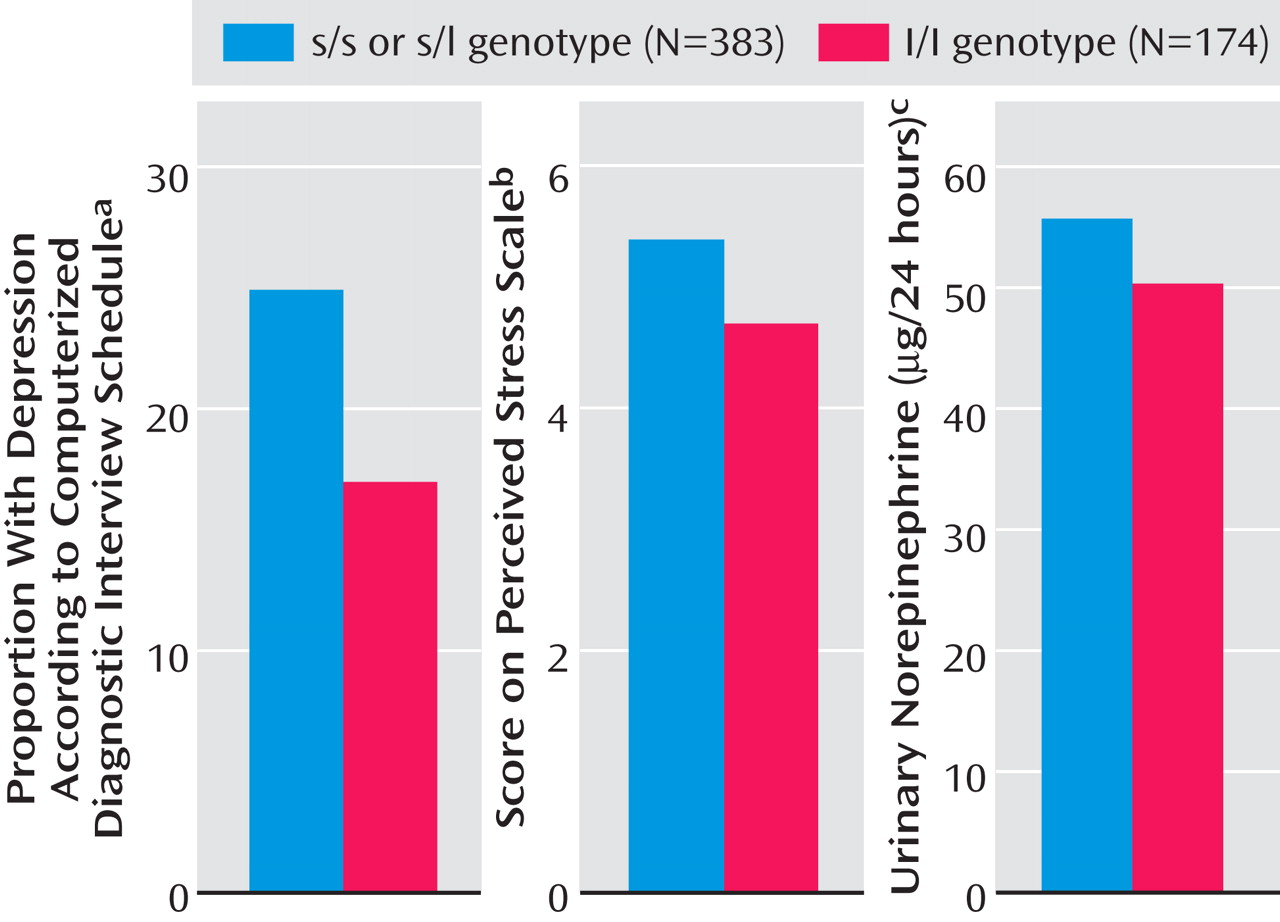
Of the 557 participants, 126 (23%) were currently depressed. The proportions of participants with current depression, our primary outcome variable, were identical in individuals with the s/s genotype (24 of 96, 25%) and s/l genotype (73 of 287, 25%), confirming a dominant effect of the s allele. Therefore, we designated the individuals with the s/s and s/l genotypes as s allele carriers. Current depression was diagnosed in 25% of the individuals carrying an s allele (97 of 383), compared with 17% of the l/l carriers (29 of 174) (
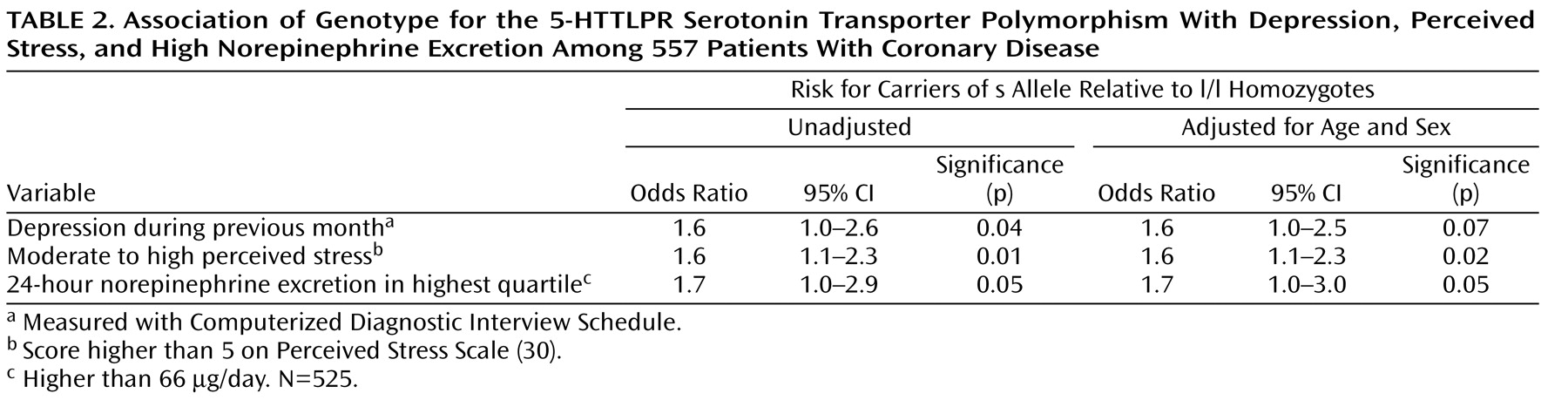
Figure 1 ); the odds ratio for depression in s allele carriers, therefore, was 1.6, as shown in
Table 2 . After adjustment for age and gender, this association remained but was not significant at the traditional p<0.05 cutoff point.
5-HTTLPR and Perceived Stress
Carriers of the s allele had a higher mean score on the Perceived Stress Scale (mean=5.4, SD=3.4) than the l/l homozygotes (mean=4.7, SD=2.9) (
Figure 1 ). In a logistic regression analysis, s allele carriers had an increased odds of having moderate to high scores for perceived stress (
Table 2 ). This association remained strong after adjustment for age and gender.
5-HTTLPR and 24-Hour Norepinephrine Excretion
Urinary 24-hour excretion of norepinephrine was higher in s allele carriers (mean=55.6 mg, SD=24.0) than in l/l homozygotes (mean=50.2, SD=23.8) (
Figure 1 ). In a logistic regression analysis, s allele carriers had higher odds of having norepinephrine values in the highest quartile (
Table 2 ).
Potential Moderating Variables
We did not find any significant effects of interactions between 5-HTTLPR and measures of cardiac disease severity, including exercise capacity (metabolic equivalents achieved), history of myocardial infarction, history of coronary artery bypass graft, or overall perceived health status in predicting depression, perceived stress, or norepinephrine excretion (all p values for interactions were >0.20).
Discussion
We found that the s allele of 5-HTTLPR was associated with depression and perceived stress in patients with coronary disease. Furthermore, the s allele was associated with greater 24-hour urinary norepinephrine excretion. These results suggest that patients with the 5-HTTLPR s allele are more likely to develop depression, greater perceived stress, and high norepinephrine secretion in the presence of chronic illness.
Our results regarding the association between the s allele and depression are consistent with previous findings of an interaction of the s allele with stressful life events in predicting depression among medically healthy subjects
(11 –
15) . We have demonstrated an association between the s allele and depression in participants who were chronically stressed because of an ongoing illness. Indeed, several small studies found an association between the s allele and depression in patients with medical illness, i.e., in patients with Parkinson’s disease
(18,
19), stroke
(20), hip fracture
(21), and coronary disease
(22) . Taken together, these findings suggest that chronic medical illness may be a type of stress that is more likely to lead to depression in patients with the s allele than in patients homozygous for the long allele.
The implication of our findings is that the 5-HTTLPR genotype may be an important vulnerability factor for depression and perceived stress in cardiac patients. Heart disease currently affects about 13 million Americans
(31) . Depression occurs in about 20% of these patients, and it is associated with an increased risk of future cardiac events and mortality
(32) . Studies have shown that s allele carriers do not respond as well as l/l homozygotes to treatment with selective serotonin reuptake inhibitors and show more adverse events
(33), making it more difficult to treat their depression. Thus, the prognosis for coronary disease might be worse for s allele carriers than for l/l homozygotes. Indeed, one study found an association of the s allele with depressive symptoms and cardiac events in a group of Japanese patients following acute myocardial infarction
(22) .
However, we did not find any moderating effects of cardiac disease severity as measured by exercise capacity, history of myocardial infarction or coronary artery bypass graft, or overall perceived health on the association between 5-HTTLPR and depression, perceived stress, or norepinephrine. Participants in the Heart and Soul Study were considerably sicker than other study groups (
Table 1 ), and small differences in disease severity may not have been sufficient to cause varying levels of stress. Grabe et al.
(34) reported that mental and physical distress was modulated by s allele status and chronic disease burden in women (but not men) in a general population sample. Future studies should explore whether greater disease severity in chronic illness moderates the association between 5-HTTLPR and depression.
Currently, the mechanisms by which the s allele might increase the risk for developing depression are unknown. The s allele has been shown to be associated with lower 5-HT
1A receptor binding potential, lower expression of the serotonin transporter and serotonin uptake in lymphoblasts, an enhanced amygdala response to fearful stimuli, stronger amygdala-prefrontal coupling, and uncoupling of an amygdala-cingulate circuit implicated in the extinction of negative affect in human imaging studies
(6) . All of these mechanisms might be relevant to the vulnerability of s allele carriers to depression.
We also found that the s allele was associated with greater 24-hour urinary norepinephrine excretion. To our knowledge, the association between 5-HTTLPR and norepinephrine has not previously been examined in humans. However, the s allele has been associated with cardiovascular reactivity to mental stress in young adult twins
(35), suggesting that individuals with the s allele have a heightened propensity to show strong reactions to stress. Moreover, knocking out the serotonin transporter gene leads to enhanced stress-induced catecholamine secretion in mice
(36) . Since norepinephrine is a risk factor for adverse outcomes in patients with coronary disease
(37 –
39), this might also be a mechanism by which s allele carriers have adverse cardiovascular outcomes.
Several limitations must be considered in our study. In our study group, all patients were chronically ill and thus were under a considerable amount of stress. To examine an effect of the interaction between the s allele and stress on depression, as reported in previous studies, an ideal study would also have evaluated a group of participants who were not exposed to the stress of chronic illness. Further, 5-HTTLPR was associated with neuroticism in a meta-analysis
(40), and we did not measure personality dimensions. It could be argued that the association with depression is caused by an underlying association with neuroticism, rather than with the diagnostic entity of depression. We were unable to examine the association of 5-HTTLPR with depression in different ethnic groups. Further, it is possible that the CDIS resulted in some misclassification of depression, since use of a standardized instrument cannot replace the diagnosis of depression by a clinician. However, nondifferential misclassification results in weaker (not stronger) associations, and unless misclassification was associated with 5-HTTLPR, it should not have affected our results. Finally, we did not measure functional variants that have been identified within the l allele and designated as l
A and l
G . The effects of the l
G allele on depression are thought to be similar to those of the s allele
(41) . Thus, genotyping for only the s and l alleles may have diluted our findings.
In summary, we found 5-HTTLPR to be modestly associated with depression, greater perceived stress, and higher 24-hour urinary norepinephrine secretion in patients with coronary disease. Individuals carrying an s allele may be at greater risk for depression in the presence of chronic illness.