Abnormalities in the frontal lobe have been widely implicated as contributing to the pathophysiology of violent behavior. The prefrontal cortex is imperative for executive functions; it is responsible for regulating inhibition, emotions, movement, self-monitoring, self-correcting, and overall social and cognitive behavior. Damage or dysfunction of the frontal lobes results in unregulated behavior. Lesion studies that date back to the late 1800s have repeatedly found that damage to the frontal lobes results in an altered personality marked by an increase in impulsivity and a lack of judgment and decision-making skills
(1 –
4) . These reports suggest that frontal lobe areas are significantly compromised and may serve as structural markers for predisposed violent behavior.
The diagnostic characteristics of antisocial personality disorder include impulsivity, failure to conform to social norms, aggressiveness, disregard for safety, glibness, and deceitfulness. Not all violent criminals have antisocial personality disorder, but there is a higher rate of severe violence among subjects with antisocial personality disorder compared to the general population. This suggests that there may be a specific pattern of cortical deficits that underlie this violent behavior. There is a lack of brain imaging data regarding the neural correlates that underlie antisocial personality disorder. However, two studies have shed some light on this topic. One study revealed that when corrected for total brain volume, individuals with antisocial personality disorder have an 11% reduction in prefrontal gray matter volume in relation to healthy comparison subjects
(5) . Another study showed that violent individuals with antisocial personality disorder, in relation to healthy comparison subjects, have a decrease in total brain and temporal lobe volumes and an increase in putamen volume
(6) .
Evidence from behavioral and functional magnetic resonance imaging (fMRI) studies further implicate frontal brain regions as contributing to the neuropathology of antisocial personality disorder. For example, a neuropsychological study by Dinn and Harris
(7) reported that individuals with antisocial personality disorder performed significantly worse than healthy comparison subjects on cognitive tasks sensitive to orbitofrontal function. Furthermore, fMRI data showed that violent individuals with antisocial personality disorder had deficits in working memory and reduced brain activations in the left frontal gyrus, anterior cingulate, and precuneus in relation to comparison subjects
(8) . Impaired emotion and behavioral inhibition, personality traits representative of antisocial personality disorder, have also been attributed to orbitofrontal dysfunction
(9) . Notably, functional imaging evidence supports that the mesial prefrontal cortex is highly involved in emotional processing and more regularly activated by emotional tasks than cognitive tasks
(10) . Thus, dysfunction of the mesial prefrontal cortex may result in abnormal emotional processing. Deceitfulness and psychopathic symptoms have also been attributed to antisocial personality disorder and frontal lobe abnormalities. Yang and colleagues
(11) have shown that gray matter volume in prefrontal regions decreases in association with higher psychopathy scores. These investigators, however, also reported that within the population with antisocial personality disorder, frontal white matter volume increases and changes in gray/white matter tissue ratios are linked with lying and deceitfulness specifically
(12) and that deficits in prefrontal gray matter are evident in unsuccessful—but not in successful—psychopaths
(11) . The high rates of violent and impulsive behavior among individuals with antisocial personality disorder and these earlier studies suggest that the prefrontal cortex or frontal lobes are compromised in violent individuals with antisocial personality disorder. This pattern of structural deficits may also be involved in the pathophysiology that underlies the violent behavior observed in schizophrenia patients.
Although to a lesser extent than antisocial personality disorder, the incidence of violent behavior in schizophrenia is greater than in the general population, at least in a subgroup of patients
(13) . Neuropathology in schizophrenia has been widely studied in which cumulative evidence suggests that multiple brain regions are affected in the disease. Specifically, schizophrenia patients have significant reductions in brain volume and weight
(6,
14) . In addition, CSF enlargements and gray matter deficits in the lateral and medial temporal cortices, principally the superior temporal gyrus and hippocampus subcortically, are reported in the large majority of studies
(14,
15) . Gray matter abnormalities in other neocortical regions, including the frontal cortices, notably the prefrontal and orbitofrontal regions and the parietal cortices, are also reported in schizophrenia
(16,
17) . Finally, several studies have shown that cortical thinning within frontal, temporal, and parietal cortical association regions is associated with disease processes in schizophrenia
(16,
18) .
The existing literature concerning the structural neuropathology of schizophrenia is vast, and the literature regarding the neural underpinnings of
violent behavior in this population is growing. In a recent review by Naudts and Hodgins
(19), the authors concluded that male schizophrenia patients who consistently showed a pattern of aggressive and antisocial behavior had poorer orbitofrontal functions, an increase in the size of the putamen
(6), and reductions in the volume of the amygdala, the hippocampus, and the orbitofrontal system compared to schizophrenia patients with no violent history. These findings suggest that there may be specific deficits in the prefrontal-limbic circuitry in violent schizophrenia patients. Notably, some studies have also shown that relationships exist between frontal white matter integrity and aggressive symptoms of schizophrenia. In a diffusion tensor imaging study, Hoptman et al.
(20) reported inverse relationships between fractional anisotropy and motor impulsiveness scores and positive relationships between diffusion tensor imaging trace measurements and aggression scores in male schizophrenia patients (N=14) in right hemisphere inferior frontal regions. In a later study, this group also reported that larger left orbitofrontal cortical gray matter volumes and larger bilateral orbitofrontal white matter volumes were associated with aggression in schizophrenia patients
(21) . Remarkably, the only fMRI study to have investigated the neural correlates of seriously violent behavior in schizophrenia so far has linked bilateral frontal and right parietal activation deficits to serious violence in schizophrenia
(8) . In the same study, activation deficits in the sensorimotor cortex, thalamus, and hippocampus were associated with violence across both antisocial personality disorder and schizophrenia.
Analyses of the anatomy of violent groups with antisocial personality disorder and schizophrenia may provide insight into the neural underpinnings of violent behavior. Barkataki et al.
(6) recently reported findings on structural/volumetric abnormalities in violent patients with antisocial personality disorder and schizophrenia. Their findings show that although violent subjects with antisocial personality disorder and schizophrenia have some common structural abnormalities, differences exist, suggesting that some brain systems are selectively involved in violent antisocial personality disorder compared to violent schizophrenia groups
(6) . Thus, in this investigation, we set out to examine regional differences in cortical thickness in an overlapping group of the subjects included in the study by Barkataki et al.
(6), namely, violent individuals diagnosed with antisocial personality disorder, violent and nonviolent schizophrenia patients, and well-matched healthy comparison subjects. There are few to no data concerning the regionally specific cortical correlates of violent behavior in antisocial personality disorder and schizophrenia, nor are there any data that indicate whether structural changes associated with violent behavior differ in these two populations. Although previous studies have explored regional changes in cortical thickness in schizophrenia patients, none of these studies has examined the effects of violence specifically. Moreover, disturbances in laminar thickness and the structural difference associated with violence have not yet been addressed in individuals with antisocial personality disorder, to our knowledge. Cortical thickness serves as an indicator of cortical integrity and provides an overall profile of anatomical and possible functional deficit. Based on existing data characterizing structural abnormalities in schizophrenia and antisocial personality disorder, we hypothesized that violent schizophrenia patients and violent individuals with antisocial personality disorder would both exhibit regional alterations in cortical thickness within frontal neocortices. In order to elucidate changes in cortical thickness specific to violent behavior and to investigate whether violence effects differed in our two violent subject groups, we used cortical pattern-matching methods to align homologous cortical regions between subjects.
Method
Subjects
The study participants included individuals with antisocial personality disorder with a history of violence (N=14), patients with schizophrenia and a history of violence (N=12), patients with schizophrenia without a history of violence (N=15), and healthy nonviolent comparison subjects (N=15). All study groups included right-handed male subjects between 18 and 55 years (mean=33.5 years, SD=10.4; mean=34.4, SD=5.2; mean=34.5, SD=7.5; mean=32.1, SD=7.5, respectively). All study groups were also matched on socioeconomic status. All magnetic resonance and neuropsychological measures were collected at the Institute of Psychiatry, London. The antisocial personality disorder group was diagnosed with antisocial personality disorder without schizophrenia comorbidity; similarly, both schizophrenia groups were diagnosed with schizophrenia without antisocial personality disorder comorbidity. Individuals with antisocial personality disorder did not present with psychopathic personality features as assessed by the Psychopathy Checklist—Screening Version
(22) . The violent subjects were recruited from a high-security hospital. Nonviolent schizophrenia patients living in the community were recruited through local London hospitals, and normal comparison subjects were recruited through local advertisements. No patients with antisocial personality disorder were medicated; however, both schizophrenia groups were receiving standard doses of antipsychotic medications.
All antisocial personality disorder and violent schizophrenia patients were free of alcohol and substance misuse for a minimum of 2 years (confirmed by regular random urine screens in secure hospitals) at the time of scanning for this study. However, past alcohol and substance misuse or dependence was relatively more common in these patients than in nonviolent schizophrenia patients. In the violent schizophrenia group, two patients had a history of alcohol dependence, one of solvent misuse, two of cannabis dependence, one of alcohol as well as polysubstance misuse (cannabis, Ecstasy, LSD, amphetamines), and one of alcohol dependence and polysubstance misuse. In the group with antisocial personality disorder, two patients had a history of alcohol dependence, one of alcohol and cannabis dependence, two of alcohol dependence and polysubstance misuse, and five of alcohol and polysubstance misuse. In the nonviolent schizophrenia group, two patients had a previous history of using cannabis and one of using cannabis and LSD (the last use was more than 10 years before taking part in this study). The comparison group had no past or present drug misuse or dependence.
Diagnoses and clinical assessments were made with the clinical interview for DSM-IV personality disorders for the group with antisocial personality disorders
(23) and the clinical interview for DSM-IV axis I disorders
(24) for the schizophrenia groups. All healthy comparison subjects were also interviewed to screen for mental illness (the SCID nonpatient interview)
(24) . In addition to assessment of mental health, all subject groups’ severity of violent history (or lack thereof) was scaled on the Gunn and Robertson scale
(25) . Our subject groups were a subset of the subjects included in a study by Barkataki et al.
(6) in which further details about subject recruitment, clinical assessment, and medication history can be found. The research ethics committees of the Institute of Psychiatry and Maudsley Hospital, London, approved all procedures, and informed written consent was obtained from all subjects. Approval for image analysis was obtained through collaborative data-sharing agreements with the Laboratory of Neuro Imaging at UCLA in accordance with UCLA institutional review board protocols.
Image Acquisition and Preprocessing
High-resolution three-dimensional T 1 -weighted spoiled gradient-recall acquisition magnetic resonance images were acquired on a 1.5-T Signa GE scanner (Milwaukee, Wis.) at Maudsley Hospital as a series of 124 contiguous axial slices (1.5-mm slice thickness without gaps, 256×256 matrix, 24-cm field of view, TR=13.8 msec, TI=450 msec, TE=2.8 msec, flip angle=20°, 6-minute 30-second acquisition time).
Image processing included a series of executable functions performed on the T 1 -weighted images including the following:
Whole-brain extraction to remove nonbrain tissue and the cerebellum
(26) . All minor errors from automated scalp-editing procedures were manually corrected in each brain volume
Correction for magnetic field inhomogeneity artifacts
(27)Correction for head tilt and alignment with a three-translation and three-rotation rigid-body transformation
(28,
29)Transformation of structural MRI into common stereotaxic space without scaling
(30)Automated tissue classification with a partial-volume classifier method in which voxels are labeled as most representative of white matter, gray matter, or cerebral spinal fluid
(31)Surface rendering that results in a three-dimensional object model representative of the shape of the cortex. For surface rendering, a spherical mesh surface is continuously deformed to fit a cortical surface tissue threshold intensity value (a signal value that differentiates extra cortical CSF and brain tissue) from the brain volume rigidly aligned in ICBM-305 space
(30)Finally, 31 sulcal landmarks were delineated manually in each hemisphere with previously validated surface anatomical protocols that were later used for cortical pattern matching procedures described below
(17,
32) . Interrater reliability of manual outlining of sulci was measured as the three-dimensional root mean square difference in millimeters between 100 equidistant points from each sulcal landmark traced in six test brains compared to a gold standard arrived at by a consensus of raters. Intrarater reliability was computed by comparing the three-dimensional root mean square distance between equidistant surface points from sulcal landmarks from one test brain traced six times by the same rater. Three-dimensional root mean square disparities were <2 mm, and on average <1 mm, between points for all landmarks within and between raters
(16) .
Cortical Pattern Matching
Cortical pattern-matching methods were used to spatially relate homologous regions of the cortex between subjects that allow for measurement and comparison of cortical thickness at homologous hemispheric locations in each individual
(17,
32) . For the matching procedures, a surface-warping algorithm computes a three-dimensional vector deformation field that records the amount of x, y, and z coordinate shift (or deformation) to associate the same cortical surface locations in each subject with reference to the average anatomical pattern of the entire study group by using the manually derived sulcal/gyral curves as landmarks
(17) . These methods reparameterize (regrid) the hemispheric surface so that the same anatomy bears the same coordinate locations at 65,536 surface points without scaling the hemispheres. In other words, the sulcal landmarks are used as anchors to drive the surrounding cortical surface anatomy of each individual into correspondence. The cortical pattern-matching methods allow for measurement of cortical thickness at thousands of homologous cortical locations in each subject in which three-dimensional coordinate locations are matched.
Cortical Thickness Estimation
The three-dimensional deformation fields obtained from the cortical pattern-matching algorithms equate homologous cortical locations between individuals, allowing a local measure of cortical thickness to be made at equivalent hemispheric surface points in each individual. In order to index gray matter thickness at higher resolution than the acquired data, the tissue-classified brain volumes were resampled at 0.33 cubic mm. With an implementation of the three-dimensional Eikonal equation
(33), cortical thickness was defined as the shortest three-dimensional distance, without crossing voxels classified as CSF, from the cortical white-gray matter boundary to the hemispheric surface (gray-CSF boundary)
(17) . In the presence of partial volume effects in which gray matter surfaces appear adjacent, distance values were progressively coded until they met a voxel already assigned a distance code. Thus, opposing gray matter banks not separated by CSF receive the same thickness value. In practice, this may underestimate or overestimate cortical thickness on one side of the sulcus. However, the thickness on the other side will be altered in precisely the opposite way. Furthermore, for comparisons across subjects, a spatial filter of radius 8 mm was applied to the coded distance values so that no practical difference could result from misattributing the thickness to one side of the sulcus or the other.
Statistical Analyses
All analyses were performed with the statistical package R (http://www.r-project.org/). The statistical model included a two- (violent, nonviolent) by-two (schizophrenic, nonschizophrenic) design to examine the main effects of violence and schizophrenia. Therefore, we increased statistical power by collapsing groups across these two dimensions and assessed the interactions between these two variables. Analyses were performed after we covaried for age and total brain volume. This model was used to examine the effects of violence and schizophrenia for total gray matter volume and other tissue compartments (white matter and CSF) and, subsequently, to examine regional cortical thickness changes between groups. In implementing the two-by-two design described above, statistical comparisons with the general linear model were made at each of the 65,536 hemispheric surface locations in three dimensions to reveal regionally specific cortical thickness changes across the cortex between subject groups defined by violence and schizophrenia. Follow-up comparisons were performed to examine the simple effects of violence between comparison subjects and individuals with antisocial personality disorder and between nonviolent schizophrenia and violent schizophrenia patients. The results of these tests are mapped onto the three-dimensional group-averaged hemispheric surface models where statistically significant results are indexed in color (
Figure 1 ). An uncorrected two-tailed alpha level of p<0.05 was determined as the threshold for statistical significance and for interpreting results.
Given our a priori hypothesis of cortical thickness alterations in the prefrontal cortices, permutation analyses were performed to confirm the significance of results for these regions of interest specifically. For permutation testing, a mask was made of the prefrontal cortex with a probabilistic atlas
(34) . The number of surface points within this region of interest that were significant at a threshold of p<0.05 was compared to the number of significant surface points within the region of interest that occurred by chance when subjects were randomly assigned to groups across 1,000 new randomized analyses. Because there are few data concerning the structural correlates of violence in antisocial personality disorder and schizophrenia, especially with regard to cortical thickness changes, results implicating other cortical regions were treated as exploratory. However, because study groups exhibited significant overall gray matter volume differences, these analyses appeared justified.
Results
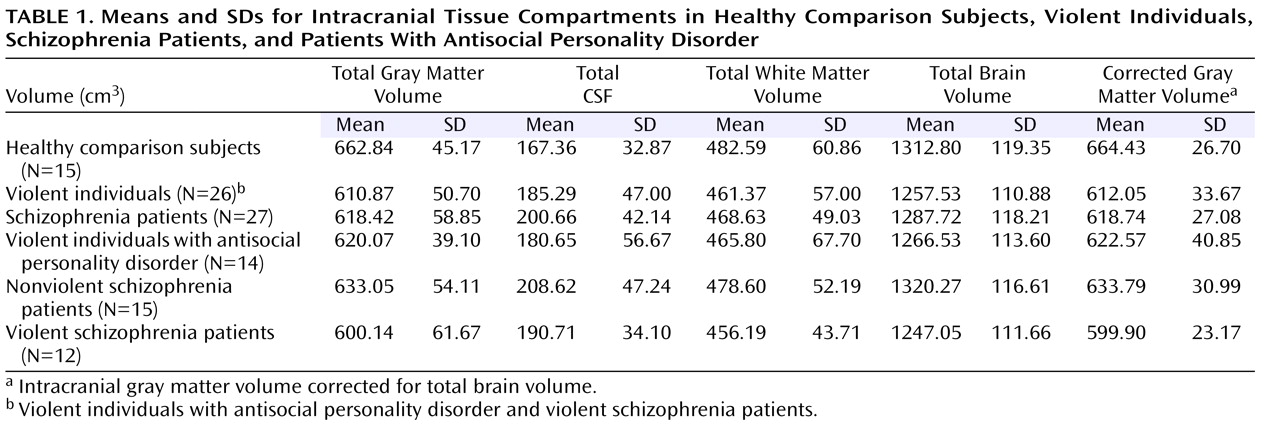
Table 1 shows the means and standard deviations for each intracranial tissue compartment (total brain, whole-brain gray matter, white matter, and CSF volumes) within study groups defined by violence and schizophrenia. After we covaried for age and total brain volume, reductions in whole-brain gray matter were observed in schizophrenia and violent individuals (F=9.67, df=1, 55, p≤0.003, and F=5.27, df=1, 55, p<0.03, respectively). There was a significant CSF effect for the schizophrenia subjects (F=6.64, df=1, 55, p<0.02). No other group differences were found for intracranial CSF or white matter (p>0.05).
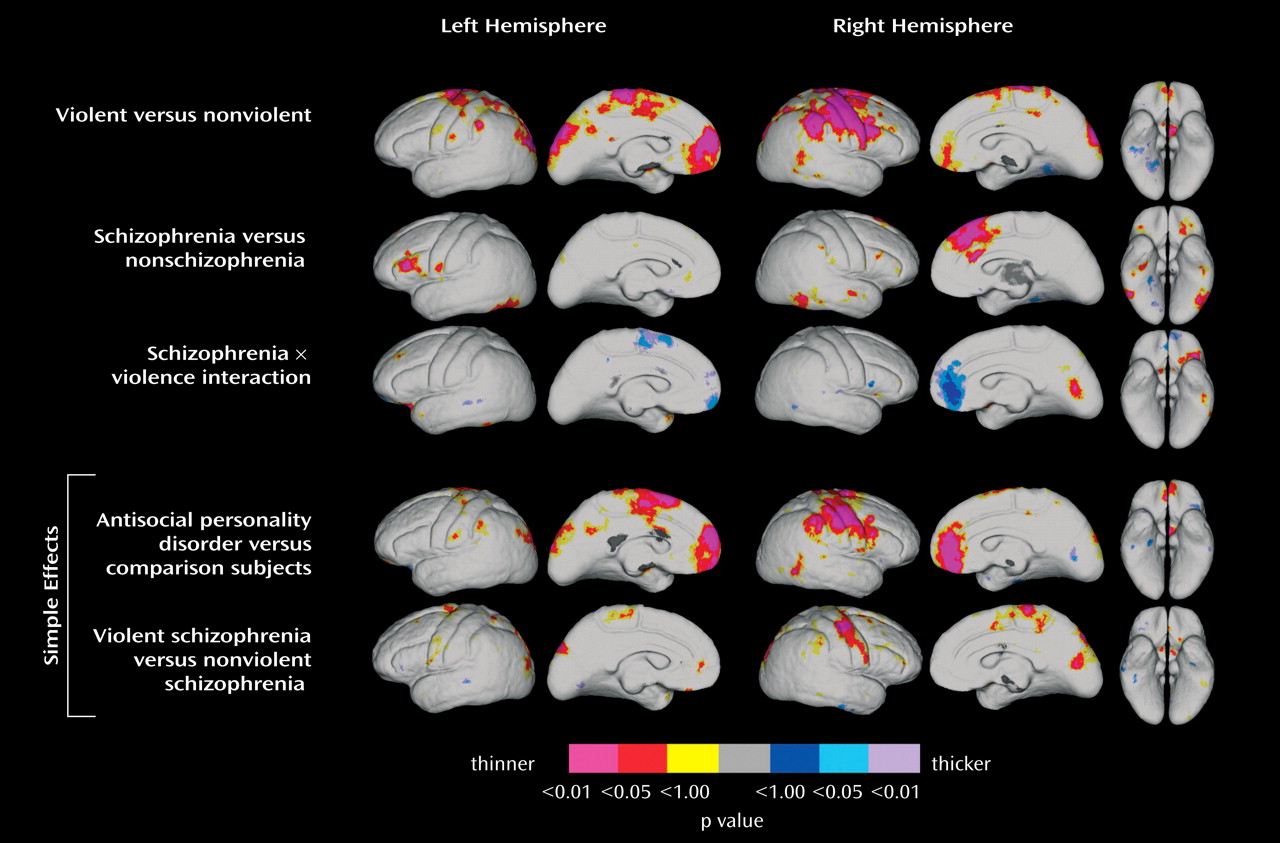
Significant regional effects of violence (violent subject groups and nonviolent subject groups) and schizophrenia (schizophrenia subject groups and nonschizophrenia subject groups) were observed for cortical gray matter thickness. Cortical thickness results are shown in
Figure 1, in which violence and schizophrenia effects are mapped in the top two rows, interaction effects are mapped in the middle row, and the bottom rows show statistical mapping results for cortical thickness comparisons between the antisocial personality disorder group and the comparison group and between violent schizophrenia and nonviolent schizophrenia patients, respectively. Significant regional differences in cortical thickness are shown in color, as indexed by the color bar in which positive and negative effects are represented by different color scales.
Statistical maps show that main effects of violence (
Figure 1, top row) are associated with thinning of the prefrontal cortex that is pronounced in the medial inferior frontal area (to a larger extent in the left hemisphere), the lateral sensory motor cortex and surrounding association areas (Brodmann’s areas 10, 11, 12, and 32; more pronounced in the right hemisphere), and in areas surrounding the posterior region of the intraparietal sulcus bilaterally in violent compared to nonviolent subjects. Schizophrenia groups (
Figure 1, second row) showed cortical thinning surrounding the inferior dorsolateral (Brodmann’s areas 46 and 45) prefrontal regions, with significant thinning in the left hemisphere compared to nonschizophrenia groups (
Figure 1, second row). In addition, this contrast revealed significant thinning of the dorsal medial frontal cortices in the right hemisphere.
Interactions between violence and schizophrenia showed changes in cortical thickness surrounding medial inferior frontal cortices in groups defined by a diagnosis of schizophrenia and by violent behavior (
Figure 1, third row). To explore these regional interactions, analyses of simple effects (antisocial personality disorder versus healthy comparison subjects and violent versus nonviolent schizophrenia patients) (
Figure 1, last two rows) confirmed that cortical thinning in the inferior medial frontal cortices is specific to violent subjects with antisocial personality disorder. Subjects with antisocial personality disorder showed significant thinning of the medial inferior frontal cortices bilaterally and thinning of sensory motor areas in the right hemisphere in relation to healthy comparison subjects. Violent schizophrenia subjects showed deficits in sensory motor areas compared to nonviolent schizophrenia subjects, as consistent with the overall effects observed for violence. Region-of-interest (frontal cortices) permutation analyses confirmed findings (p<0.05) for the violence effect and for the contrast between antisocial personality disorder and comparison subjects in the right hemisphere.
Discussion
Cortical thickness is related to the packing density, number, and size of neurons. A decrease in cortical thickness thus implies a compromise in structure because of changes in the characteristics and integrity of the neuropil. Cortical thickness on magnetic resonance imaging may be related to regional neuronal density in the cortical mantle, but it may also depend on unknown vascular factors, glial cell numbers, and the extent and integrity of cortical myelination
(35) . This measure, therefore, may be sensitive to white matter as well as to gray matter characteristics, in which the extension of myelinated fibers into the cortical mantle can account for cortical thinning. Reductions in cortical thickness may thus implicate weak neuronal connectivity associated with deficits in information processing. In this investigation, we examined regionally specific differences in cortical thickness across subject groups defined by a diagnosis of schizophrenia and by a history of violent behavior. Our main objective was to investigate the structural correlates of violent behavior and to determine whether such effects differ in antisocial personality disorder and schizophrenia. We found main effects of schizophrenia in lateral inferior frontal and dorsal medial frontal cortices that are partially consistent with prior findings
(16,
18) . Main effects in the patterns of cortical thickness were also observed in direct association with violent behavior as assessed by comparing subjects with a violent history (patients with antisocial personality disorder and violent schizophrenia patients) and nonviolent subjects (healthy comparison subjects and nonviolent schizophrenia patients). Violent subjects exhibited significant bilateral cortical thinning within inferior medial frontal cortices, pronounced thinning of the sensorimotor cortex in the right hemisphere, and thinning in discrete areas of the motor cortex in the left hemisphere. In addition, violent subjects showed significant bilateral thinning in the regions surrounding the intraparietal sulcus. Notably, varying patterns of cortical thickness abnormalities were associated with violent behavior in individuals with antisocial personality disorder and schizophrenia. That is, the interaction effect between violence and schizophrenia and subsequent analyses of simple effects showed that cortical thickness changes in medial frontal cortices are specific to individuals with antisocial personality disorder with a history of violent behavior. In contrast, the pattern of cortical thinning associated with violent behavior in schizophrenia exclusively was observed only in the sensorimotor cortex.
Our findings contrast with a prior report showing positive relationships between scores of aggression and bilateral orbitofrontal cortex white matter volume and left orbitofrontal cortex gray matter volume in schizophrenia or schizoaffective disorder
(21) ; only sensorimotor regions showed significant differences in cortical thickness when we compared violent and nonviolent schizophrenia subjects in our study. These inconsistencies in results may be attributable to a number of factors, including statistical power issues in which intersubject variation in cortical thickness measurements may render small effects in orbitofrontal cortex regions below the threshold of significance. However, differences in demographic and clinical features of the study groups, including the substance abuse profiles and the methods used to characterize violence in general, may have contributed to differences in findings. Finally, volumetric measurements from regions of interest may or may not relate to cortical thickness measures obtained at extremely high spatial resolution across the cortex. Overall, our findings suggest that although the violent behavioral symptoms are similar between our violent antisocial personality disorder and schizophrenia subject groups, the underlying pathology contributing to this behavior may be different.
Implications of Prefrontal Cortical Thinning Associated With Violence
Cortical thinning in the medial prefrontal cortex observed in violent individuals with antisocial personality disorder is consistent with the existing literature regarding the primary roles and dysfunction of the prefrontal cortex and supports our a priori hypotheses of disturbances in the structural integrity of this region that may underlie these subjects’ propensity for violence. Violent behavior is a common symptom of many mental illnesses. For example, impulsive and aggressive behaviors, commonly associated with assault and suicidal behavior, are characteristic of borderline personality disorder. In a 2003 positron emission tomography study, Soloff et al.
(36) reported that patients with borderline personality disorder had a decrease in fluorodeoxyglucose uptake in the medial orbital frontal cortex (orbitofrontal cortex)
(36) . In other words, patients with borderline personality disorder with violent personality characteristics have medial orbitofrontal cortex (Brodmann’s areas 9, 10, and 11) dysfunction. Furthermore, there have been several prior reports of lesions in the medial prefrontal cortex in humans and animal models suggesting that damage to this particular region of the frontal lobe often results in deregulation of affect, impulsivity, and social behavior and impaired decision-making skills, while leaving other cognitive functions intact
(2 –
4) . In an imaging study examining cognitive appraisal of negative emotion, normal subjects showed increased neural activation in the medial prefrontal cortex when performing self-focused (as opposed to situation-focused) down-regulation of emotion
(37) . These findings suggest that this region of the prefrontal cortex is involved in self-assessment and self-referential judgments
(38) . In addition, they suggest that structural abnormalities of the prefrontal cortex, as seen in our violent individuals with antisocial personality disorder, are related to disruption of self-monitoring and self-control. The medial prefrontal cortex has also been shown to be involved in anticipating anxiety
(39) . Therefore, if these neocortical regions are impaired in a subject, their ability to gauge consequential punishment for violent behavior may also be compromised. Thus, with support from the existing literature on function and dysfunction of the prefrontal cortex, our results suggest that regional cortical thinning of the prefrontal cortex is directly associated with our violent antisocial personality disorder subjects’ predisposition toward extreme violent behavior.
Implications of Somatosensory Cortical Thinning Associated With Violence
In addition to cortical thinning in the mesial prefrontal cortex, violent individuals with antisocial personality disorder exhibited significant cortical thinning in lateral sensorimotor and surrounding association cortices. Regional effects were also observed in right hemisphere sensorimotor cortices in violent schizophrenia patients. Violence-related cortical thinning in the sensorimotor cortex may represent a dysfunctional somatic loop
(40) . The somatic marker hypothesis posed by Damasio
(40) asserts that decision making involves the influence of somatic states marked by bioregulatory processes and includes a neural circuitry involving the ventromedial prefrontal cortex (orbitofrontal cortex and mesial prefrontal cortex), limbic regions, and somatosensory cortices. The hypothesis suggests that an emotional mechanism rapidly activates a mental process that recognizes the prospective consequences of an action; based on these consequences, an action is performed. Therefore, when parts of this structural circuit are dysfunctional, as in our violent subjects, the appropriate emotionally induced somatic responses may not be triggered and disadvantageous decisions or actions may be made.
Impaired Decision Making Due to Deficits in Emotional Arousal
Bechara et al.
(41) investigated the performance of patients with damage to a node in the somatic marker hypothesis circuit—specifically, the ventromedial frontal lobe—using a decision-making gambling task. Although ventromedial frontal lobe patients learned the task correctly, they repeatedly chose the least advantageous option. Investigators concluded that because the ventromedial frontal lobe was damaged, these patients were incapable of generating the necessary somatic signals when making a decision. Their data also showed that ventromedial frontal lobe patients were not emotionally aroused during the task, and this affected their performance
(41) . The involvement of the ventromedial frontal cortex in risky decision making with the gambling task was confirmed by an fMRI study that showed a positive relationship between medial prefrontal cortex activity and the more risky choice
(42) . Furthermore, in support of the role of emotionally induced visceral reactions in decision making, in 2001, Loewenstein et al.
(43) proposed an hypothesis termed “risk as feelings” that highlights the role of emotions in risky decision making. Loewenstein et al. emphasized the role of anticipatory emotions or immediate visceral reactions in making decisions. A prior study by Raine et al.
(44) lends further support for our hypothesis that violent subjects suffer from inappropriate emotional arousal due to deficits in essential parts of the somatic marker hypothesis circuit (namely, the medial prefrontal cortex and the somatosensory cortex). These investigators reported that low autonomic arousal in adolescents was predictive of criminal behavior in adulthood
(44) . Taken together, these data suggest that because of a deficit in regions in the somatic marker circuit (namely, medial prefrontal and somatosensory cortices), our violent subjects had compromised visceral emotional response when making decisions, and this insufficient autonomic response may be predictive and correlative of violent decisions. The cortical thickness patterns exhibited in violent individuals with antisocial personality disorder, and to a lesser extent violent schizophrenia patients, fits well with the somatic marker hypothesis of Damasio
(40) and “risk as feelings” by Loewenstein et al. The deficit in cortical thickness measured in two regions of Damasio’s posited circuit
(40), namely, the medial prefrontal cortex and somatosensory cortices, may further explain the pathology that underlies these subjects’ impulsive and aggressive behavior.
Implications of Motor Cortical Thinning Associated With Violence
The thickness abnormalities in the motor cortex may be related to functional deficits in motor activity, but neuropsychological data suggest otherwise. The Adult Memory and Information Processing Battery includes a set of critically timed number-cancellation tasks that provide measures of motor speed, cognitive speed, and processing accuracy. Motor speed performance is assessed by the number of cancelled items within a given time limit on a noncognitive task. In a study conducted by Barkataki et al.
(45), neuropsychological measures were compared among violent schizophrenia and nonviolent schizophrenia patients, violent individuals with antisocial personality disorder, and well-matched healthy comparison subjects. The authors reported a significant group effect in motor speed performance, tested behaviorally
(45) . However, no difference in motor speed was observed in the contrasts in which we found the greatest cortical thickness differences, namely, between the violent antisocial personality disorder and comparison groups and between the violent schizophrenia and nonviolent schizophrenia patients. Nevertheless, our findings of these specific deficits in cortical thickness are well supported by a previous study by Kumari et al.
(8) . In their sample, overlapping with that of this study, a sensorimotor cortex activation deficit was present in both the violent schizophrenia and antisocial personality disorder groups during the control condition (mainly requiring eye-hand coordination) but not in the nonviolent schizophrenia group
(8) . These data, along with our findings of motor cortical deficits in both of our violent populations, suggest that these deficits are directly involved in aggressive or violent behavior. Future studies of the regulation of motor behavior or imaged motor activity in these individuals may thus help to further explain the pathology that underlies their violent behavior.
Effects of Schizophrenia
When we examined the overall effects of schizophrenia, patients with schizophrenia, irrespective of violent history, possessed a significantly thinner cerebral cortex in the right medial dorsal anterior cingulate (Brodmann’s areas 8, 6, 32, and 24) and the left lateral inferior frontal cortex (Brodmann’s area 45) in relation to healthy comparison subjects and subjects with antisocial personality disorder. Although these findings appear less pronounced and spatially diffuse than some previous observations of cortical thickness abnormalities in schizophrenia, these findings may reflect reductions in statistical power associated with employing a smaller although well-matched study group and/or influenced by collapsing healthy comparison subjects and individuals with antisocial personality disorder in this omnibus comparison. Our results are in line with prior reports of cortical thickness reductions in frontal heteromodal association cortices
(16,
17) . Indeed, the structural integrity of many different brain regions are implicated in schizophrenia, and there is some disagreement concerning the regional specificity and magnitude of the structural changes associated with the disorder
(14,
16 –
18) . Notably, schizophrenia studies rarely characterize—and may not include—schizophrenia subjects with a history of violence. Our failure to detect more widely spread cortical thickness abnormalities in schizophrenia may also be attributable to other unique clinical characteristics of patients. Nonetheless, our findings suggest that cortical pathology in schizophrenia may be largely similar between schizophrenia patients who are violent and nonviolent. Schizophrenia is a complex illness, and violent behavior is just one manifestation of the underlying pathology. However, a particular structural deficit within motor cortices in schizophrenia patients may be associated with a predisposition to violent behavior.
Conclusion
The two violent populations of interest in this investigation possess similar histories of violent behavior, but regionally specific variations in cortical thickness show that the anatomical substrates, and thus potentially the cortical networks underlying this behavior, are at least partially different in antisocial personality disorder and schizophrenia. Violence-associated cortical thinning in the medial prefrontal cortices appears exclusive to antisocial personality disorder and may reflect a disruption of functional systems affecting judgment, emotion, self-monitoring, and impulsivity. Violence-related cortical thinning within motor cortices appears to overlap in antisocial personality disorder subjects and in schizophrenia patients with a violent history, particularly in the right hemisphere. The involvement of mesial frontal and sensorimotor cortical thinning in antisocial personality disorder may also reflect disturbances in the somatic cortical loop. Disturbances in this system may therefore underlie, to some extent, the pattern of cortical thinning observed in motor cortices in violent schizophrenia subjects. The schizophrenia literature suggests that a broad network of brain regions contribute to the underlying pathology of the illness, but this is the first study, to our knowledge, to provide evidence that the motor cortex is of particular importance to violent behaviors within this disease.