Offspring of parents with major depressive disorder face threefold greater risk for major depressive order than offspring without such family histories
(1) . Neural correlates of this familial risk have been minimally studied. A full understanding of neural correlates of the risk of major depressive disorder requires the study of at-risk individuals before they have passed the developmental period of risk. Since major depressive disorder typically first emerges in early adulthood
(2), data from at-risk adults without histories of major depressive disorder may reflect neural correlates of resilience as opposed to risk. To our knowledge, no prior study has examined the neural correlates of risk for major depressive disorder in unaffected juvenile offspring of parents with major depressive disorder through functional magnetic resonance imaging (fMRI).
Since major depressive disorder is a disorder of emotion, neural correlates may be best understood in the context of emotional processes. For example, adults with major depressive disorder show perturbed responses to motivationally salient stimuli. Clinically, they ruminate more about negative events and report less satisfaction from rewards than healthy peers
(3,
4) . In the laboratory, they show perturbed information processing of motivationally salient stimuli
(5 –
8) .
The amygdala and nucleus accumbens respond to negative and positive signals. The amygdala is a rapid detector of cues affecting well-being
(9) . Across various species, the amygdala responds to positive and, most reliably, negative stimuli, such as fearful faces
(9 –
13) . The nucleus accumbens, a structure within the ventral striatum, is most consistently responsive to rewards, such as money and happy facial displays
(14 –
20) . The nucleus accumbens also regulates motor responses to aversive stimuli
(19) . Together the amygdala and nucleus accumbens mediate detection and reaction to motivating stimuli.
Patients with major depressive disorder show perturbed activation in these structures. In adult major depressive disorder, fearful and sad faces elicit greater amygdala activation than in comparison subjects
(21,
22) . Data are less consistent in youth. In a small report, fearful faces produced relatively decreased amygdala activation in girls with major depressive disorder
(23) . In contrast, a larger study found enhanced amygdala activation in adolescents with major depressive disorder relative to healthy adolescents during incidental memory encoding of faces
(24) . In adults with major depressive disorder, there is relatively heightened activation in the nucleus accumbens to sad faces
(22,
25) and reduced activation to happy faces
(22) . Moreover, youth with major depressive disorder showed reduced activation in multiple regions, including the striatum, in response to a monetary reward
(26) . These studies have examined patients with major depressive disorder rather than individuals at risk. To our knowledge, there are no reports of amygdala or nucleus accumbens correlates of familial risk for major depressive disorder. However, relatedly, research in adults has linked amygdala function to genetic variation. Individuals with short alleles on the serotonin transporter gene are at increased risk for major depressive disorder
(27) and show hyperactivation of the amygdala in response to fearful faces
(28) .
The present study uses fMRI to examine juvenile offspring of adults with major depressive disorder and of healthy adults while they viewed faces varying in intensity of happy, neutral, and fearful expressions. Rather than solely presenting prototypical expressions, we incrementally varied affective intensity with morphing software to optimize the detection of differences in neural responses
(29) . Based on prior work, we probed the amygdala and nucleus accumbens
(21 –
25,
28,
30) . We used happy faces to engage reward processing and fearful faces to engage threat processing. We used fearful faces, as opposed to angry or sad faces, because fearful faces reliably activate the amygdala
(9 –
11,
31) . Furthermore, as noted above, neuroimaging investigations of genetic-based risk for major depressive disorder in adults yield reliable between-group differences in amygdala activation with fearful faces
(28) .
Brain activation is also influenced by attention and cognition. Tasks with low cognitive demands, such as passive viewing of emotional stimuli, preferentially engage subcortical neural circuits
(10,
11,
32) . On such tasks, adults with major depressive disorder show greater striatal and amygdala activation than comparison subjects
(22) . Tasks with greater cognitive demands do not engage these structures consistently
(10,
11,
32) . In a prior study, behavioral responses of offspring of parents with major depressive disorder and comparison subjects to face-processing tasks did not differ
(33) . The normal behavioral performance of juveniles at risk for major depressive disorder indirectly suggests that cognitive attentional demands may normalize neural perturbations in at-risk offspring. Therefore, we hypothesized that high-risk offspring, relative to low-risk offspring, would show 1) greater activation in the amygdala and nucleus accumbens in response to fearful faces and 2) less activation in the nucleus accumbens to happy faces. Moreover, we hypothesized 3) that these differences would be more pronounced in low- relative to high-demand cognitive conditions.
Method
Participants
The National Institutes of Mental Health and New York University School of Medicine’s institutional review boards approved the procedures; written informed consent was acquired from parents and offspring ages 18 years and older. Offspring below 18 years of age signed assents.
The participants consisted of 17 offspring of parents with major depressive disorder (high-risk group) and 22 comparison subjects (low-risk group). High-risk status was based on lifetime history of major depression in at least one parent.
The parents with major depressive disorder had been patients at mood and anxiety disorder clinics. They received the Structured Clinical Interview for DSM-III-R (SCID)
(34), administered by trained clinicians. The parents of low-risk offspring were similarly evaluated and found to be free of a lifetime history of anxiety, mood, or psychotic disorders.
Offspring were ages 10 through 18 years, with IQs greater than 70. Offspring were evaluated for lifetime mental disorders through direct interviews and interviews with parents about the offspring by blinded trained clinicians with the Parent as Respondent Informant Schedule (PARIS) interview
(35) . A diagnosis required either the parent or offspring report to confirm full diagnostic criteria. Exclusion criteria for all offspring included any lifetime history of major depressive disorder, any current psychiatric disorder, and current use of any psychoactive substance. Although all offspring with current psychopathology were excluded, those with past disorders (except for major depressive disorder) were not. Pubertal status was not ascertained.
Diagnostic interviews of parents with depression and their offspring were conducted in New York. Interviews of parents without depression and their offspring were conducted in either New York or Maryland.
Task Procedures
All fMRI data were collected in Maryland. fMRI probed brain function while the subjects viewed faces that varied in intensity of happy or fearful expression and neutral faces. Specifically, the fearful or happy face was morphed with the neutral face of the same model in 25% increments
(29) . The subjects viewed 10 types of facial displays: 100% happy, 75% happy/25% neutral, 50% happy/50% neutral, 25% happy/75% neutral, 100% neutral, 25% fearful/75% neutral, 50% fearful/50% neutral, 75% fearful/25% neutral, 100% fearful, and exaggerated fear (150%). Facial displays were presented in random order across subjects. By parametrically modulating the faces across levels of expression, the degree of facial emotion and brain activation can be correlated.
The subjects viewed 80 different faces (eight models by 10 levels of emotion) for a total of three viewings, once in each of three attention conditions (described below). Each attention condition was presented in eight blocks of 10 pictures each from the 80 picture set. The order of the three attention conditions was randomly assigned across subjects, and stimuli were randomly assigned across subjects and across blocks. Faces were displayed for 3 seconds, with intertrial intervals varying between 750 and 1250 msec.
Facial expression varied randomly from trial to trial (event-related) and the attention condition alternated every 10 trials (block). During face viewing, the subjects alternated across three attention conditions
(11) . In one condition, the subjects passively viewed the faces (attention was unconstrained). In another condition, the subjects attended to their subjective fear while viewing the emotional and neutral faces. In the third attention condition, the subjects attended to a nonemotional feature of the face (nose width). The subjects were cued to the condition with an instruction screen that appeared for 3 seconds. For the passive viewing condition, the instructions stated, “Just look straight ahead. Do not rate the next set of faces.” For subjective fear, the subjects were directed to press a five-key button box responding to “How afraid are you? 1. Not at all. 2. Just barely. 3. A little. 4. Very. 5. Extremely.” For nose width, the subjects were directed to press a five-key button box to indicate “How wide is the nose? 1. Not at all. 2. Just barely. 3. A little. 4. Very wide. 5. Extremely wide.” Before scanning, a practice session trained the subjects until they demonstrated appropriate performance.
Neuroimaging Procedures and Analyses
We used a GE Signa 3-tesla scanner (General Electric, Milwaukee) to acquire 29 interleaved 3.3-mm axial slices parallel to the anterior commissure-posterior commissure plane (echo-planar single shot gradient echo T 2 * weighting, TR=2300 msec, TE=23 msec, field of view=240 mm, 64×64 matrix, 3.3×3.75×3.75 mm voxel). High-resolution T 1 -weighted volumetric scans used an magnetization prepared rapid gradient echo (180.10-mm axial slices, field of view=256 mm, number of excitations=1, TR=11.4 msec, TE=4.4 msec, matrix=256×256, TI=300 msec, bandwidth 130 Hz/pixel=33 kHz for 256 pixels in-plane resolution=1 mm 3 ).
Functional imaging data were analyzed with SPM2. Data underwent slice-timing correction to adjust for temporal differences in slice acquisition within each volume. Data were motion-corrected to the first functional volume and spatially normalized to a Montreal, Neurologic Institute T
1 -weighted template image. The normalization of juvenile brains to a standard brain template is considered valid
(36) . Data were then smoothed with a 6 mm full-width at half-maximum Gaussian kernel.
Functional data were analyzed first at the individual subject level and then at the group level with the general linear model. At the subject level, a design matrix was specified for each subject with one basis function per condition. The regressors were derived from a 3-second rectangular pulse that was convolved with a synthetic hemodynamic response function from SPM2. Regression coefficients corresponding to the conditions were estimated from each subject’s blood-oxygenation-level-dependent response with the design matrices. With these coefficients, parametric contrasts were generated within each attention condition by assigning weights to each face depending on the intensity of happiness or fear expressed
(29,
37) . The linear trend analysis modeled responses from happy to fearful (including neutral). Specifically, a linear trend analysis from happy to fearful faces with all data compared neural changes as a function of changing stimulus intensity.
Next, the individual subject parametric contrasts were submitted to random-effects group-level analyses. We used a small volume-correction procedure with a threshold of p<0.05
(38) with regions of interests for the amygdala and the nucleus accumbens. Boundaries for these structures are described elsewhere
(11,
14) . As a first step, we performed an F test on the two risk groups, the three attention conditions, and the parametrically varied faces. This provided an omnibus test of the hypothesis concerning group-by-attention-by-face-emotion interactions for the amygdala and the nucleus accumbens. The overall F value was followed by t tests on the parameterized emotional faces for the three attention conditions separately to identify the factors that contributed significantly to the between-group differences
(29) . Finally, to further characterize the results and to make it possible to relate them to nonparameterized emotional face studies, we conducted post hoc t tests on the nonchimeric faces (i.e., 100% happy, neutral, 100% fearful) using the peak voxels of activation from the t tests of the parameterized faces.
Behavioral Data Analyses
Behavioral responses of fear and nose ratings as well as reaction times were submitted to repeated-measures analyses of variance (ANOVAs). Responses that occurred after 3 seconds were not recorded. Greenhouse-Geisser corrections for violations of sphericity were applied when necessary.
Results
Group Characteristics
The high-risk group, nine boys and eight girls, had a mean age of 14.3 years (SD=2.1) and a mean IQ of 102.9 (SD=13.4). The low-risk group, 10 boys and 12 girls, had a mean age of 13.9 years (SD=2.5) and mean IQ of 105.8 (SD=10.0). There were no group differences. High-risk offspring were recruited from a larger study at the New York University Child Study Center of the biology of risk for anxiety and depressive disorders. Nine low-risk comparison subjects were drawn from the larger study, and to reduce travel costs, 13 additional comparison subjects were recruited from Maryland.
Of the high-risk offspring, 10 of 17 (59%) had a past history of an anxiety disorder, and two (1.2%) had a previous history of attention deficit hyperactivity disorder (ADHD), one with oppositional defiant disorder. Of the low-risk comparison subjects, three of 22 (14%) had a history of an anxiety disorder. Separate analyses were conducted with the 26 low- and high-risk subjects without past anxiety and ADHD/oppositional defiant disorder (see data supplement available online at http://ajp.psychiatryonline.org).
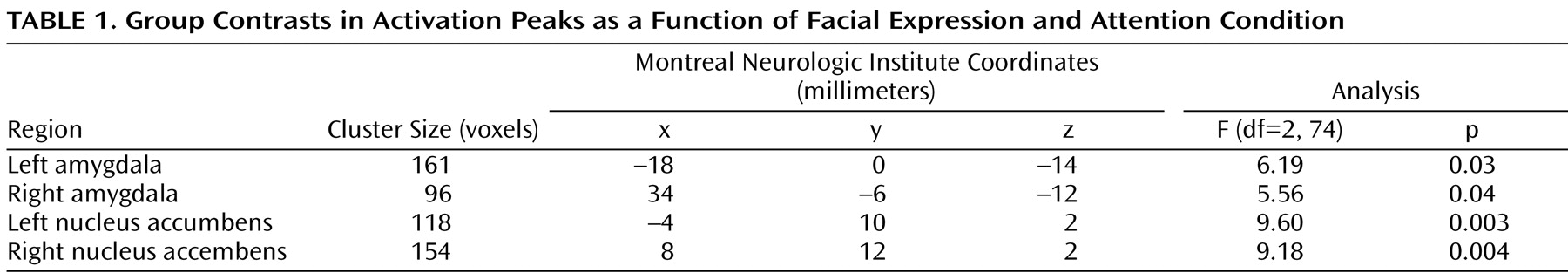
Hypothesis 1: High-risk offspring relative to low-risk comparison subjects show significantly greater activation in the amygdala and nucleus accumbens in response to fearful faces.
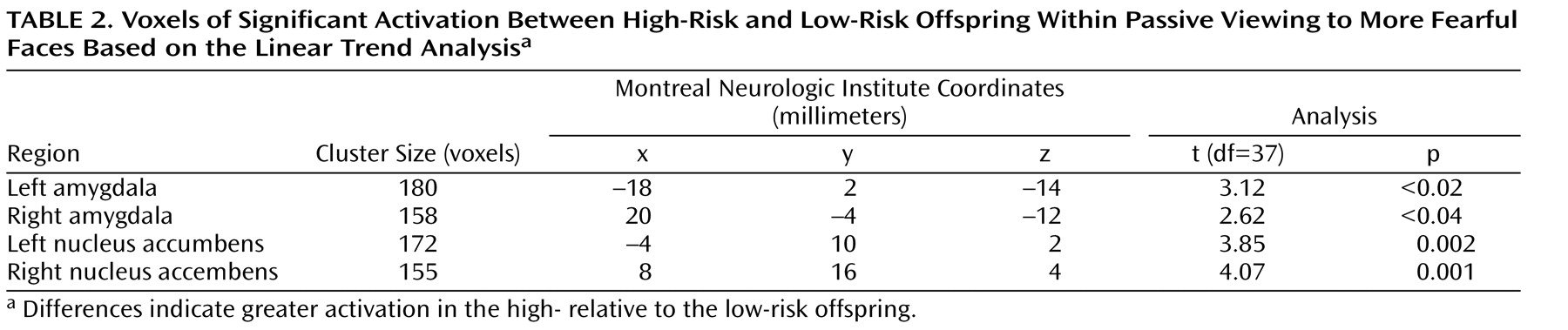
As noted above, a three-step analytic process tested this hypothesis. First, the overall F value revealed significant activation differences bilaterally in the amygdala and nucleus accumbens between the high- and low-risk groups (
Table 1,
Figure 1 ). Second, separate t tests for each of the three attention conditions, with linear trend analyses, indicated that in the passive viewing condition, high-risk relative to low-risk offspring showed greater bilateral activation in the amygdala and nucleus accumbens to the more fearful faces (
Table 2,
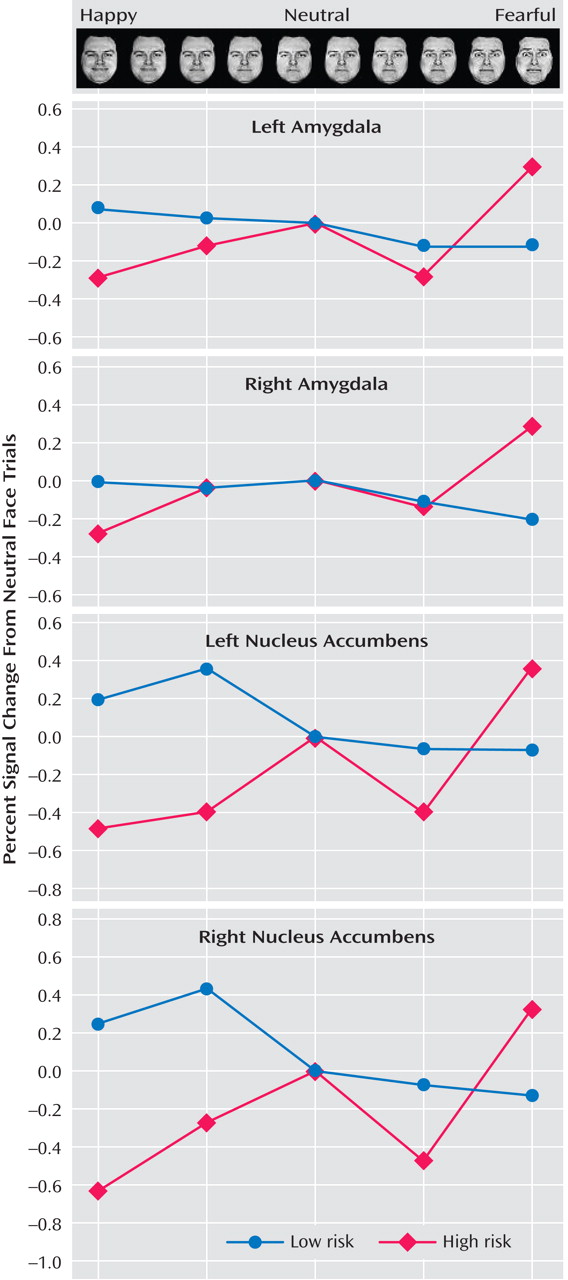
Figure 2 ). To illustrate these associations, contrast values across the facial expressions are plotted (
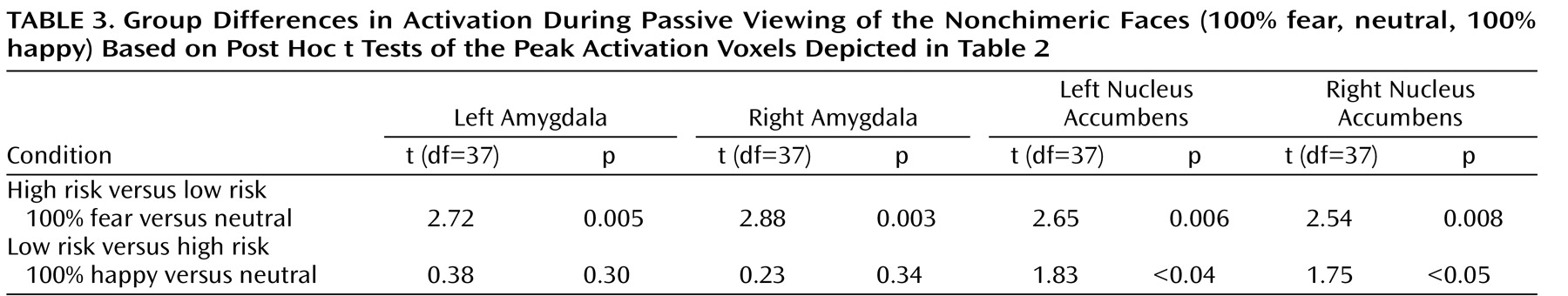
Figure 3 ). Third, post hoc t tests were conducted to characterize group differences within passive viewing. Relative to low-risk, high-risk offspring showed greater bilateral amygdala and nucleus accumbens activation during the passive viewing of 100% fearful faces than of neutral faces (
Table 3 ).
Hypothesis 2: High-risk offspring relative to low-risk comparison subjects show significantly less activation in the nucleus accumbens to happy faces.
Compared to low-risk juveniles, high-risk subjects had less bilateral nucleus accumbens activation during the passive viewing of happy faces (100%) than of neutral faces (
Table 3,
Figure 3 ).
Hypothesis 3: There is an interaction between risk status and attention condition on amygdala and nucleus accumbens activation such that increased amygdala and nucleus accumbens activation during passive viewing of fearful faces in the high-risk group is significantly reduced by attentional demands of the cognitive tasks.
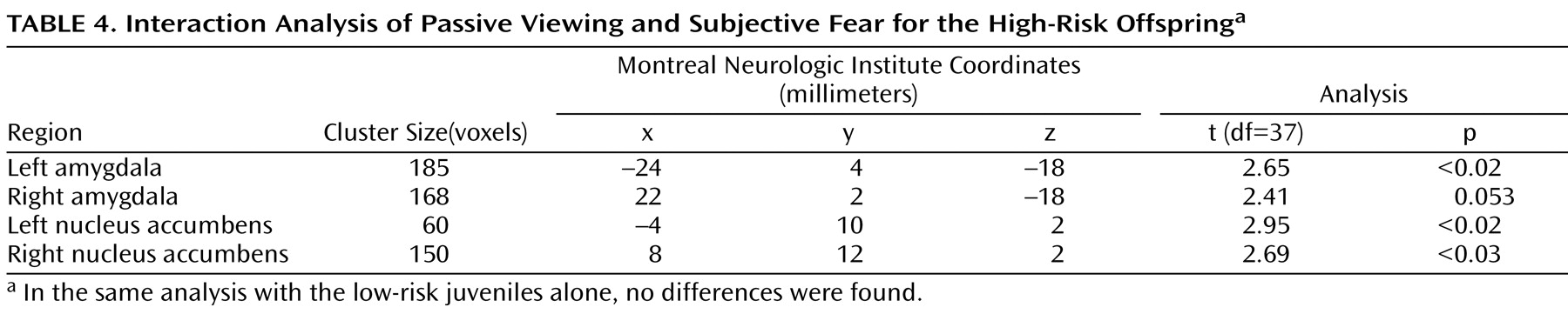
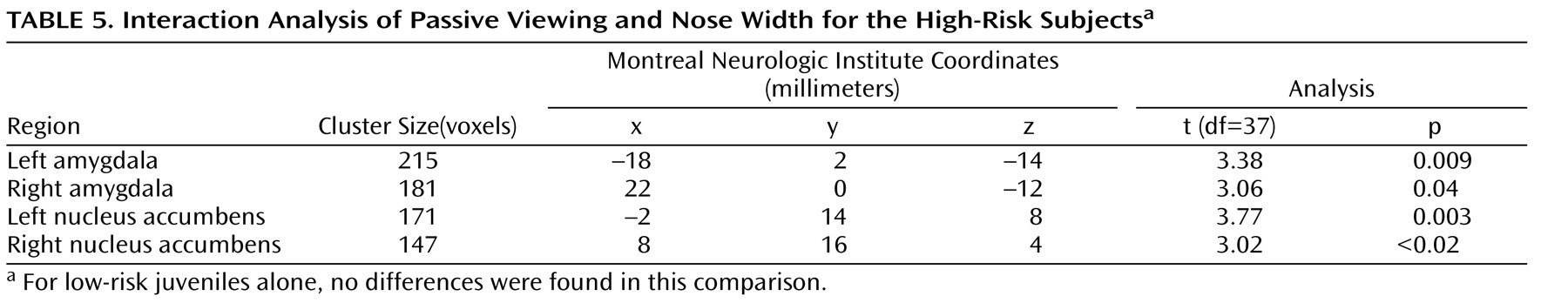
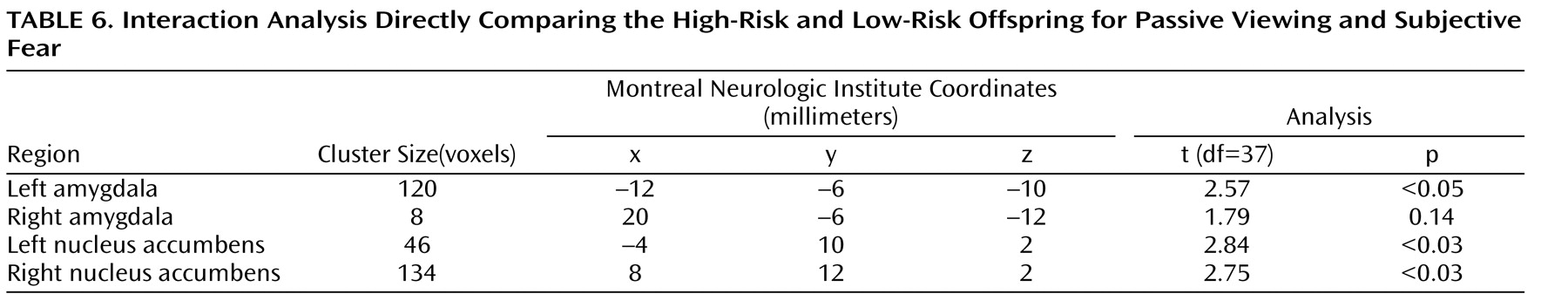
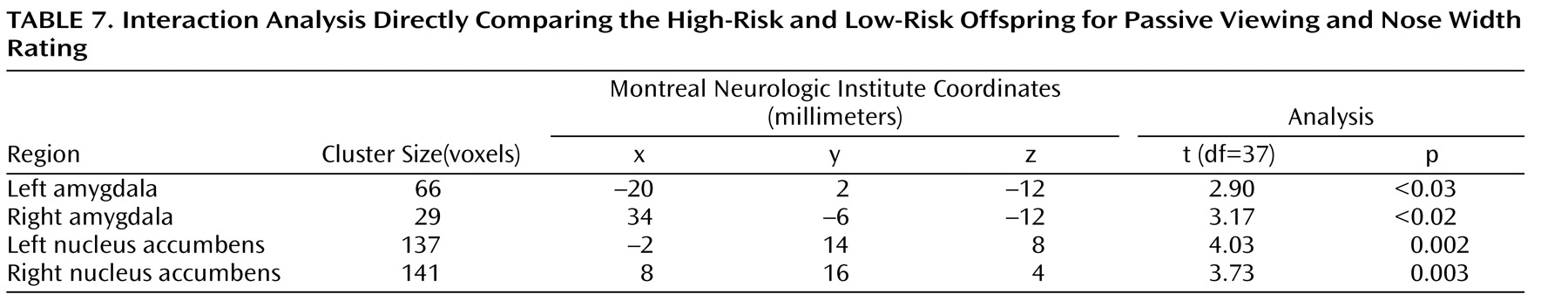
To test this hypothesis, we performed an interaction analysis of the passive viewing condition and each attention condition (i.e., passive viewing versus subjective fear and passive viewing versus nose width) with the linear trend analysis. Direct group contrasts showed that the increase in bilateral amygdala and nucleus accumbens activation during passive viewing relative to the constrained attention conditions was greater in the high-risk than the low-risk offspring (
Table 4,
Table 5,
Table 6,
Table 7 ).
Within the high-risk offspring, there was greater activation bilaterally in the amygdala and nucleus accumbens during passive viewing than during rating of fear and of nose width (
Table 4,
Table 5,
Table 6,
Table 7 ). Within the low-risk group, there were no activation differences between passive viewing and the active attention conditions. These findings suggest that the attention conditions may engage other structures, such as areas within the prefrontal cortex, that are thought to inhibit abnormal activation in the amygdala and nucleus accumbens. If so, we would expect high-risk subjects to show greater prefrontal cortex activation to fearful faces in the two attention-constraining tasks compared to low-risk subjects. Exploratory analyses yielded results consistent with this prediction. Compared to low-risk youth, high-risk subjects showed significantly greater medial prefrontal cortex activation to fearful faces when we combined the two attention conditions (subjective fear and nose width rating) and examined group differences (t=2.91, df=37, p=0.003, uncorrected, cluster size=951; x, y, z coordinates=–2, 34, 0). This was the only area of activation that surpassed a threshold of 0.05 uncorrected. No group differences were found in this area when attention was unconstrained.
Additional Findings
Further analyses addressed six additional considerations. First, analyses of task performance indicated that the subjects performed as expected (i.e., the subjects reported little to no subjective fear to happy faces and higher subjective fear to fearful faces) in both risk groups, with no group differences (data supplement Table 1). Second, we examined whether group differences in fMRI results remained when subjects with previous anxiety were removed. The same amygdala and nucleus accumbens group differences emerged (data supplement Table 2). Third, we evaluated whether the removal of the two high-risk offspring with past diagnoses of ADHD and oppositional defiant disorder affected the results. The results were unchanged, with one minor exception (data supplement Table 2). Namely, group differences in right amygdala activation during passive viewing of fearful faces became a tendency when the small volume correction was applied (p=0.065). Fourth,
Figure 3 fails to document amygdala activation in low-risk juveniles to fearful faces in one specific contrast. In supplemental analyses for other contrasts, expected amygdala activation to fearful faces in both low- and high-risk juveniles was found, with no between-group differences (data supplement Table 3). Fifth, we found no evidence that activation differed as a function of gender, but subgroups were small. Sixth, age did not relate to activation linearly or curvilinearly.
Discussion
To our knowledge, this is the first study to examine amygdala and nucleus accumbens correlates of familial risk for major depressive disorder. Three principal findings emerged. First, offspring at high risk for major depressive disorder relative to those at low risk showed greater amygdala and nucleus accumbens activation to fearful faces. Second, high-risk relative to low-risk offspring evidenced less nucleus accumbens activation to happy faces. Third, group differences in the amygdala and nucleus accumbens to emotional faces were found only during passive viewing and not during constrained attention. Exploratory analysis indicated that constraining attention recruited the medial prefrontal cortex to a greater degree in the high-risk than in the low-risk group. This may possibly account for the normal amygdala and nucleus accumbens activation when attention was constrained.
The finding that the risk groups differed only during passive viewing suggests that neural processes may be regulated, in part, by attention in those at high risk for major depressive disorder. When attention is constrained, responding is comparable in high- and low-risk subjects. Thus, constraining attention may mobilize top-down cortical systems that normalize function in subcortical circuits
(10,
11) . This possibility is suggested by the finding that attention tasks engaged greater medial prefrontal cortex activation to fearful faces in the high-risk than in the low-risk offspring. Meanwhile, the passive viewing condition may allow the expression of neural and related mental processes, such as rumination, that reveal aberrant neural activation associated with vulnerability to major depressive disorder.
Findings in the present study are consistent with adult neuroimaging findings in patients with major depressive disorder but differ from a small study
(23) in which five girls with major depressive disorder, ages 8–16, showed attenuated amygdala activation to fearful faces. Sampling and methodological differences may account for discrepant findings. Among these, the previous study used a block design in which a specific facial expression was presented repeatedly at a high rate. Therefore, reduced amygdala activation may have been due to amygdala habituation to repeated presentation of fearful faces
(39) . In this study, the presentation of facial expressions changed from trial to trial, minimizing habituation.
Limitations
Although there was a strong rationale for studying fearful faces, the absence of other negative expressions, such as sad faces, precludes knowing whether activation differences between offspring at high- and low-risk for major depressive disorder are specific to fear stimuli or occur across negative emotions. If effects were selective to fear, it would provide evidence of specific perturbations. Another limitation concerns the inclusion of offspring with a history of anxiety. However, since anxiety disorders are common in offspring of parents with major depressive disorder, the exclusion of past anxiety would have reduced the representativeness of the group, thereby decreasing the generalizability of the findings. At the same time, the frequent occurrence of anxiety disorders in the high-risk group may limit clear interpretation of the findings. In this study, lifetime anxiety disorders did not account for differences between juveniles at high and low risk for major depressive disorder (data supplement Table 2), indicating that group differences in activation were a function of the risk status of major depressive disorder and were not related to the presence of anxiety disorders. In addition, the present study was not designed to address developmental changes in brain function and how they interact with the risk for psychopathology. It will be important for future studies to characterize the role of the amygdala and the nucleus accumbens from a developmental perspective as well as in relation to vulnerability for mood disorders. Finally, it was not possible to determine whether group differences in brain function were mediated by differential attention to specific facial expressions. Nevertheless, the findings document that each group showed increases in response to some stimuli and decreases in response to others during passive viewing. These results are inconsistent with the possibility that one group failed to attend during passive viewing.
Future Directions
First, replication is necessary. It will be particularly important to study larger groups of children at risk for major depressive disorder with and without anxiety to examine the contribution of anxiety disorders to neural perturbations in at-risk individuals. Second, the inclusion of multiple negative emotional stimuli, such as sad and angry faces, would inform on the specific significance of emotional stimuli. Third, while offspring of parents with major depressive disorder are at greater risk for the disorder, they are also at increased risk for other mental disorders, especially anxiety disorders
(1) . Thus, the present findings may not relate to the risk of major depressive disorder specifically. Longitudinal studies would inform this question and also whether variations in neural activation predict the incidence of major depressive disorder among those at high risk. Such work could eventually identify which of the at-risk individuals are at highest risk for major depressive disorder and other psychopathology. For example, individuals with the highest activation in a given structure or a particular neural interaction profile might be those at highest risk. If so, the study of prevention efforts would be especially appropriate in this subgroup of at-risk individuals. Fourth, the study of eye movements might identify patterns of gaze that enhance brain activation in high- versus low-risk subjects during passive viewing.