Epidemiology
PD is the second most common neurodegenerative disease after Alzheimer’s disease, affecting up to 1% of the elderly population. DLB, which may be indistinguishable from PD neuropathologically and has similar clinical features (e.g., dementia, spontaneous parkinsonism, and attentional impairment), is now thought to be the second most common dementing illness after Alzheimer’s disease in Western countries. Dementia also occurs commonly in PD, affecting up to 75% of PD patients over the long term
(1) . The distinguishing clinical characteristic between PD with dementia and DLB is the timing of dementia onset: dementia that occurs with, at the time of, or within 1 year of the onset of parkinsonism is diagnosed as DLB, whereas a dementia onset more than 1 year after the onset of parkinsonism is diagnosed as PD with dementia
(2) .
Psychotic disorders (or psychosis) occur rarely in untreated PD patients
(3) ; those with comorbid late dementia, depression, or delirium are at the greatest risk
(4) . In contrast, illusions or hallucinations occur in 15%–40% of treated PD patients
(5 –
8) . Up to 10% of patients experience delusions, usually in addition to hallucinations
(6,
8) . In one study
(9) of 289 consecutive PD outpatients, 18% had hallucinations only, 7% had hallucinations plus “confusion,” 4% had hallucinations plus delusions, and 2% had delusions only. Persistent psychotic symptoms in PD are associated with greater functional impairment, caregiver burden, and nursing home placement
(6,
10,
11) .
Recurrent visual hallucinations are a core feature of DLB, and other types of hallucinations, as well as systematized delusions, are supportive features for a diagnosis of DLB
(2) . Thus, in contrast with PD, psychosis may occur in DLB regardless of treatment with dopamine replacement therapies or other antiparkinsonian treatments. Approximately 75% of DLB patients experience hallucinations, and more than 50% have delusions
(12) . In a comparative study
(11) of patients with DLB, PD with dementia, and PD without dementia, both delusions and hallucinations were most common in the DLB group (57% and 76%, respectively), followed by the PD with dementia group (29% and 54%, respectively) and then the PD without dementia group (7% and 14%, respectively).
Presentation
Patients with psychotic disorders associated with parkinsonism fall into two phenomenological groups. One group typically experiences mild visual perceptual changes (e.g., sensation of a presence or a sideways passage) or visual illusions or visual hallucinations only, although auditory hallucinations and, more rarely, olfactory and tactile hallucinations can also occur
(5,
13) . Visual hallucinations are typically formed animal or human figures and are stereotyped for each patient. PD patients with this type of psychosis typically retain insight into the hallucinations, do not find them troubling (and sometimes do not even report the symptoms to a physician), and may not require treatment (i.e., “benign hallucinosis”).
The other group, typically PD patients with dementia and the majority of DLB patients, experiences complex psychotic symptoms, including both hallucinations and systematized persecutory delusions in the context of dementia, sometimes complicated by delirium
(6) . In a comparative study of PD with dementia and DLB
(12), the most common delusions in both disorders were of persecution and theft, phantom boarders, television characters in the room, and spousal infidelity; only patients with DLB experienced Capgras’s syndrome (delusional misidentification syndrome). These patients typically do not have insight into their psychosis, often find their hallucinations frightening, may display behavioral changes (including “sundowning” and other forms of agitation), and typically require treatment.
Vivid perceptions in multiple modalities and illogical thinking also occur as part of REM sleep behavior disorder, which is characterized by the loss of muscle atonia or physiological paralysis of muscles normally associated with REM sleep periods and the resultant physical and verbal acting out of dreams. Further confusing matters is that the overwhelming majority of psychotic PD patients also report sleep disturbances
(16) . Clinically, it is difficult to distinguish nocturnal psychotic symptoms from sleep-related perceptual disturbances, particularly when hallucinations occur when a person falls asleep (hypnagogic transition period) or wakes up from sleep (hypnapompic transition period).
Etiology and Pathophysiology
Exposure to PD medications (e.g.,
l -dopa, dopamine agonists, monoamine oxidase [MAO] inhibitors, anticholinergics, and amantadine) has been implicated as a major cause of psychosis in PD
(17) . A hierarchy for the propensity of specific agents to induce psychosis has been proposed, but there is limited controlled research to support this view
(13) . Both dopamine agonists and
l -dopa, the two first-line treatments for the motor symptoms of PD and the most commonly used, have been associated with psychosis
(18), and randomized comparative studies have found that psychosis occurs more commonly with dopamine agonists than with
l -dopa
(19) .
Despite the clear association between medication exposure and PD psychosis, some studies have reported that the dosage and duration of antiparkinsonian treatment are not correlated with psychosis
(8), indicating that PD psychosis likely results from a complex interaction of medication exposure, PD pathology, aberrant REM-related phenomena, and comorbid vulnerabilities, particularly cognitive impairment and visual disturbances.
Dopaminergic medication exposure may lead to excessive stimulation or hypersensitivity of mesocorticolimbic D
2 /D
3 receptors and induce psychosis
(20) . A serotonergic/dopaminergic imbalance has also been hypothesized
(13,
17,
20) . However, the association between psychosis and cognitive impairment suggests more widespread brain involvement including other neurotransmitter systems and neural pathways. Cholinergic deficits and Lewy body pathology in acetylcholine-producing neurons in the brain are common in both PD with dementia and DLB
(21), and these deficits may be associated with the occurrence of psychosis in both disorders
(22) .
In DLB, visuoperceptual impairment has been reported to be a correlate of, and possible risk factor for, psychosis
(2) . In addition, increased numbers of Lewy bodies in the temporal lobe and the amygdala, areas implicated in the generation of complex visual images, have been found to be associated with the onset and presence of visual hallucinations
(23) . Finally, brain perfusion imaging in DLB has demonstrated reduced occipital uptake in areas identified as part of the visual cortex
(2) .
Assessment
The diagnosis of psychosis in PD and DLB is based on a clinical interview, and it is often helpful to include an informed other in this process. There are no validated instruments for the assessment of psychosis in PD and DLB, but the Neuropsychiatric Inventory
(24) has been extensively used in both epidemiological and treatment studies of psychosis in these two populations. In recently proposed diagnostic criteria for psychosis in PD, to meet criteria a patient must have at least one of the following: illusions, false sense of presence, hallucinations, or delusions
(25) .
The treatment of psychosis in the context of PD is challenging, since optimizing the management of motor symptoms with dopaminergic medication typically worsens psychosis, and treating psychosis with an antipsychotic can worsen parkinsonism. A thorough evaluation for possible medical causes of delirium should be performed, and any nonessential non-PD medications that might contribute to mental impairment should be discontinued. Next, the risk-benefit ratio of each antiparkinsonian medication should be reviewed. Recommendations based on clinical experience
(13,
26) include discontinuing medications, if doing so does not make motor symptoms intolerable, starting with dopamine agonists, anticholinergics, amantadine, and MAO inhibitors, and reducing the
l -dopa dosage only if other measures fail.
Treatment of PD Psychosis
In conjunction with the steps outlined above, psychopharmacological treatment is initiated for persistent and problematic psychosis. Conventional antipsychotics are not recommended for use in patients with PD, as they have been reported to significantly worsen the motor aspects of parkinsonism
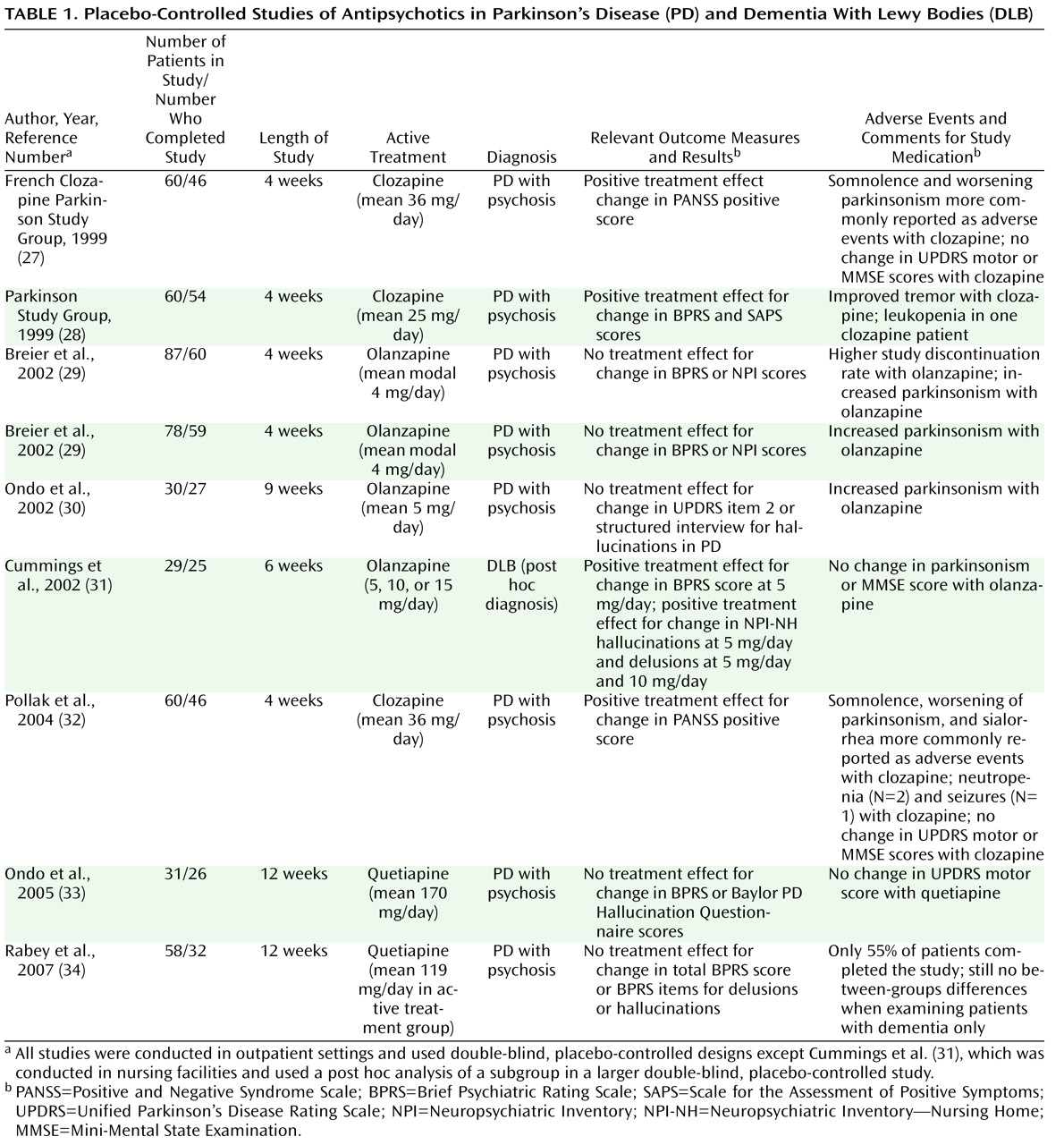
(13) . All randomized placebo-controlled studies of treatment for psychosis and related psychiatric disturbance in PD and DLB have tested either antipsychotics or cholinesterase inhibitors (
Table 1 ).
In the three adequately designed placebo-controlled studies of low-dose clozapine for the treatment of PD psychosis reported to date, clozapine was found to be significantly better than placebo in reducing positive symptoms of psychosis, with no worsening in parkinsonism or global cognition on average
(27,
28,
33) . Two reports on results of three placebo-controlled studies of olanzapine indicated no between-groups differences in efficacy, and olanzapine was associated with significant worsening of parkinsonism in all three studies, with a higher dropout rate in one study
(29,
30) . Finally, there have been two placebo-controlled trials of quetiapine, which anecdotally is the first-line treatment for psychosis in PD. In the first study, no significant differences between quetiapine (flexible dosage up to 200 mg/day) and placebo were reported for any efficacy measures, and no worsening of parkinsonism was found with quetiapine treatment
(33) . In the other study, quetiapine (mean dosage of 119 mg/day) was not efficacious for the treatment of PD psychosis, and only 55% of patients completed the study
(34) .
There have also been several antipsychotic comparator studies of treatment for PD psychosis, all involving clozapine. In a small randomized active comparator study of risperidone versus clozapine, only the risperidone group demonstrated improvement on the Brief Psychiatric Rating Scale (BPRS) psychosis cluster score, but the between-groups differences were not significant; neither group demonstrated a change in the Unified Parkinson’s Disease Rating Scale motor score
(35) . In a randomized, double-blind comparison of olanzapine and clozapine, the study was terminated early because of exacerbated parkinsonism in olanzapine-treated patients
(36) . Finally, in a randomized, single-blind active comparator study of quetiapine versus clozapine, both groups experienced significant improvement in total BPRS score with treatment, with no between-groups difference, and neither group had a worsening in Unified Parkinson’s Disease Rating Scale motor score
(37) . Summarizing the literature on antipsychotics in PD, a recent structured review and meta-analysis concluded that “only clozapine can be fully recommended for the treatment of drug-induced psychosis in PD”
(38) .
Aripiprazole, an atypical antipsychotic with partial agonism at D
2 receptors, held promise as an antipsychotic in PD when it was first introduced, but two small open-label studies at varying dosages reported negative findings overall, with a significant percentage of patients discontinuing treatment because of worsening parkinsonism or lack of improvement in psychosis
(39,
40) . In a small case series of ziprasidone at a low dosage (20–40 mg/day) for the treatment of PD psychosis, 83% of the patients completed 12 weeks of treatment, with a significant overall improvement in total Neuropsychiatric Inventory score and no change in the Unified Parkinson’s Disease Rating Scale motor score
(41) .
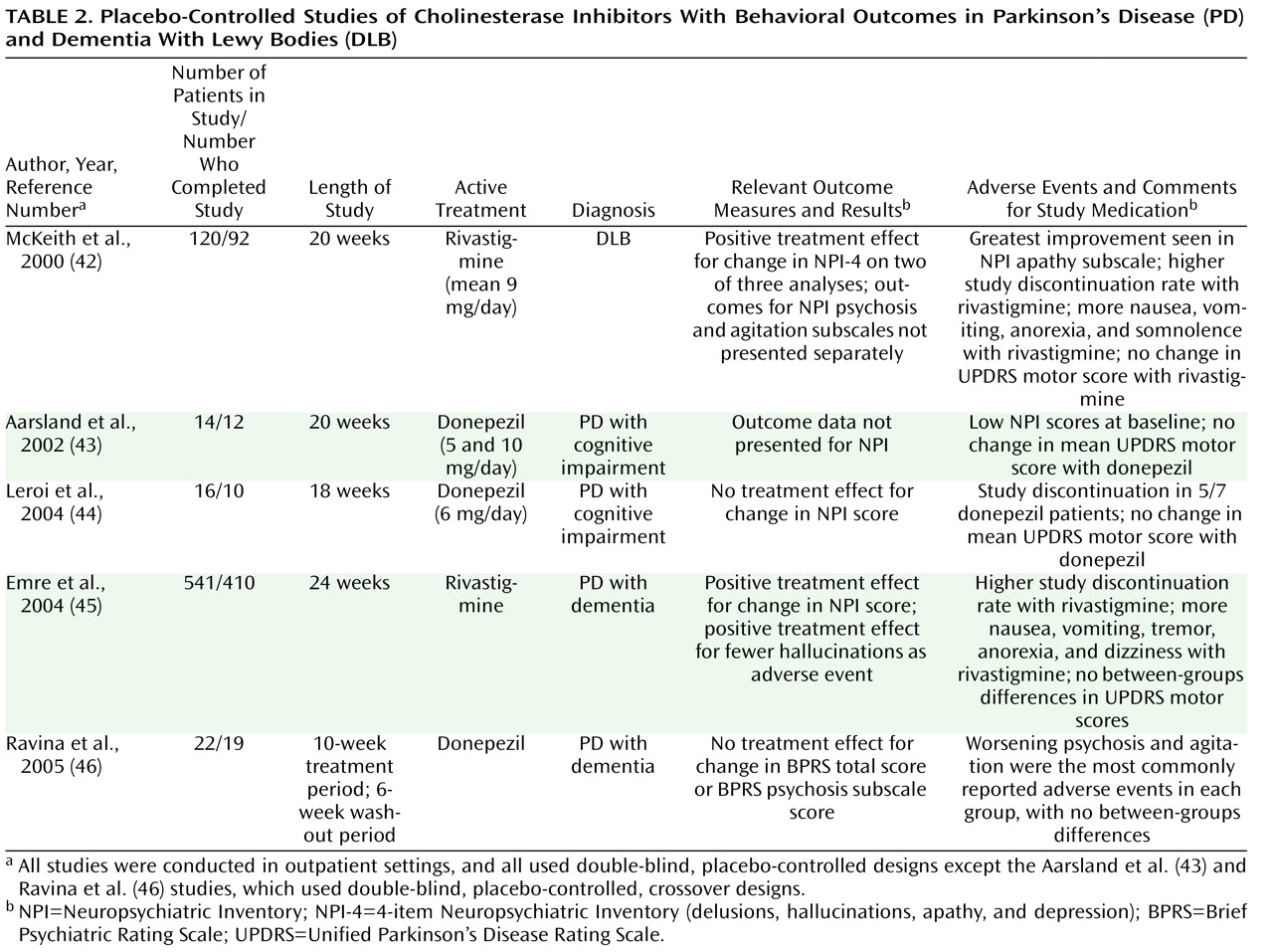
There is some evidence that cholinesterase inhibitors may have antipsychotic properties in PD (
Table 2 ). Several small open-label studies have found donepezil and rivastigmine to be beneficial for PD psychosis in the context of dementia. Three placebo-controlled studies of cholinesterase inhibitors for the treatment of PD with dementia did not demonstrate superiority for donepezil relative to placebo for psychiatric symptoms, including psychosis; however, all studies were small, were designed to evaluate cognitive outcomes, and enrolled only participants with limited psychiatric comorbidity
(43,
44,
46) . A large placebo-controlled study of rivastigmine for PD with dementia found that the rivastigmine group had a significantly greater decrease in neuropsychiatric symptoms than the control group and were less likely to report hallucinations as an adverse event
(45) . In a secondary analysis, the greatest benefit from treatment with rivastigmine was found to occur in patients who had visual hallucinations at baseline
(47) . There are also case reports suggesting that antidepressants may have a role in the treatment of psychosis in PD.
Treatment of DLB Psychosis
Only one prospective randomized study for the treatment of neuropsychiatric symptoms in DLB has been reported to date
(42) (see
Table 2 ). Rivastigmine led to significant improvement in most analyses on a composite neuropsychiatric score that included delusions, hallucinations, depression, and apathy, although it was not clear whether significant between-groups differences were seen for either delusions or hallucinations alone. In addition, the presence of visual hallucinations was found to predict improvement in attention
(48) . Cholinesterase inhibitors are the recommended first-line treatment for DLB because of their benefit for cognitive symptoms
(49) and relative lack of toxicity compared with antipsychotic medications. In a small retrospective case series of memantine for DLB, the authors concluded that this agent can be used safely in this population, but approximately one-third of patients experienced a worsening in psychiatric or cognitive status with treatment
(50) .
In a placebo-controlled study of the treatment of psychosis in Alzheimer’s disease with olanzapine, a post hoc analysis of a subgroup of patients who were retrospectively diagnosed with DLB showed that olanzapine led to a decrease in positive symptoms of psychosis without worsening either parkinsonism or cognition
(31) (see
Table 1 ). A small open-label study of quetiapine for psychosis and agitation in DLB found that approximately half of patients treated with 25–75 mg/day of quetiapine experienced a clinically significant reduction in these symptoms, but several patients had to discontinue treatment because of somnolence or orthostatic hypotension
(51) .
Safety and Tolerability
In PD, concerns about worsening parkinsonism are paramount. Clozapine and possibly quetiapine, at lower dosages, do not appear to worsen motor symptoms, although higher dosages of either agent may cause excessive sedation or confusion
(37,
52) . Results from randomized studies suggest that olanzapine tends to worsen the motor symptoms of PD
(29,
30) . The only randomized study of risperidone in PD found worsening parkinsonism with risperidone and an improvement with clozapine treatment, although the between-groups differences were not significant
(35) . The results from open-label studies with risperidone in PD have been mixed, but this drug is rarely used in treating psychosis in PD because of the perception that it worsens motor function.
One of the suggestive features for a diagnosis of DLB is sensitivity to antipsychotic medications
(2), both conventional agents
(53) and atypical agents
(54) . There have been case reports of a syndrome characterized by sedation, immobility, rigidity, postural instability, falls, and increased confusion, which has been hypothesized to be induced by the antidopaminergic and anticholinergic properties of antipsychotics. Worsening extrapyramidal symptoms (e.g., muscle rigidity and cogwheeling) are also common side effects of antipsychotic treatment in DLB, particularly with exposure to conventional antipsychotics
(55) .
In addition to these concerns about the adverse effects of atypical antipsychotics in PD with dementia and DLB, a range of other relevant adverse events and outcomes have been reported with the use of these agents in other populations, particularly in older patients with Alzheimer’s disease and other types of dementia
(56) . The labeling for all atypical antipsychotics is required to include a boxed warning for increased mortality (due to heart-related events and infections, particularly pneumonia) in older patients with dementia-related psychosis. Clozapine labeling includes in addition a boxed warning for myocarditis, agranulocytosis, seizures, and orthostatic hypotension. Several of the atypical antipsychotics also include warnings regarding cerebrovascular events (including stroke) and hyperglycemia or diabetes mellitus.
A negative impact of cholinesterase inhibitors on parkinsonian motor scores might be predicted because of their procholinergic effect on the normal balance with dopamine in the basal ganglia. Increased tremor as a self-reported adverse event was significantly more common with cholinesterase inhibitor treatment in the studies of rivastigmine in PD with dementia
(45) and DLB
(42), although motor scores on the Unified Parkinson’s Disease Rating Scale did not worsen in either study. In both studies, certain adverse events (nausea, vomiting, anorexia, somnolence, and dizziness) occurred significantly more often with active treatment, contributing to a higher rate of study discontinuation among treated patients.
Summary and Recommendations
Psychosis is very common in PD, particularly in patients with dementia, and it is a core feature of DLB. While dopamine replacement therapy likely plays a role in the development of PD psychosis, the etiology appears to be multifactorial. Impairments in the monoaminergic and cholinergic neural circuitry induced by disease-related neuropathological changes help explain the high prevalence of psychosis in both PD and DLB. Appropriate management of psychosis should begin with an evaluation of existing PD medications and withdrawal of offending agents. Clozapine is the only antipsychotic shown to be efficacious for the treatment of PD psychosis, although quetiapine is more frequently used because of convenience. Concerns about antipsychotic sensitivity in DLB patients may prevent antipsychotics from being tested further in this population. There is also preliminary evidence that cholinesterase inhibitors may have antipsychotic properties in both PD with dementia and DLB.
Screening for and treating psychosis in PD is important, as psychotic symptoms are associated with worse outcomes for patients and significant distress for caregivers. Screening can be done quickly in a routine clinic visit by inquiring about perceptual changes, particularly the range of visual disturbances that can occur. Patients should be treated with an atypical antipsychotic only if the symptoms are problematic, after acute medical conditions have been ruled out, and after the antiparkinsonian medications have been reviewed. It is important to monitor for worsening parkinsonism whenever the antipsychotic dosage is increased.
Based on open-label studies and clinical experience, the current first-line antipsychotic treatment is quetiapine (starting dosage, 12.5–25 mg/day; mean maintenance dosage, approximately 75 mg/day; dosage range, 25–300 mg/day). Although clozapine is the only antipsychotic with established efficacy for the treatment of psychosis in PD, it is best reserved for patients who do not tolerate or respond to quetiapine, because of the legal requirement for frequent blood testing to monitor for agranulocytosis. Although cholinesterase inhibitors cannot currently be considered a first-line treatment for psychosis in PD, they may have antipsychotic effects in PD, and their mild cognition-enhancing properties are likely to be of benefit to the large percentage of psychotic patients with comorbid cognitive impairment or dementia.
The natural course of psychosis in PD is not well understood, so there is little empirical evidence to guide clinicians on the appropriate length of treatment for PD psychosis. In one case series, an attempt was made to slowly wean psychiatrically stable (i.e., no longer psychotic) patients off clozapine or quetiapine, but the study was terminated early after 83% of patients experienced a recurrence of psychosis, including several whose symptoms were worse during the recurrent episode than in the initial episode
(57) . There is preliminary evidence suggesting that it is possible to switch psychiatrically stable patients from clozapine to quetiapine, provided patients have not previously failed quetiapine treatment and there is no gap in antipsychotic coverage
(58) . This strategy might make sense particularly for patients who are in the first year of clozapine treatment, given the burden of having weekly or biweekly blood drawings.
The cholinesterase inhibitors are the only class of psychiatric medications that have been evaluated in randomized clinical trials for treatment of DLB. These agents appear to have positive effects on both cognition and neuropsychiatric symptoms, including psychosis. Given the possibility of sensitivity reactions, antipsychotic medications, particularly quetiapine and clozapine, should be used cautiously in DLB patients, after a thorough discussion of the risk-benefit ratio with both the patient and an informed other, and with close clinical monitoring for adverse effects.
For the patient in the vignette, the follow-up evaluation discussion with the patient and family focused initially on making another attempt to modify the patient’s PD medication regimen. After consideration of the available options in this approach (e.g., again decreasing the dopamine agonist dosage but this time also making a concomitant increase in the l -dopa dosage or adding a catechol O -methyltransferase [COMT] inhibitor, an MAO inhibitor, or amantadine), it was felt that each option would potentially lead to either a worsening of parkinsonism or an increase in psychotic symptoms. Hence, the focus shifted to adding an antipsychotic medication. Consideration was given to restarting quetiapine at a lower dosage and titrating upward very slowly, but the severity of the current psychosis and uncertainty about the efficacy of quetiapine for psychosis in PD led the physician to recommend a trial of clozapine. The adverse event profile, the need for ongoing blood monitoring, and the overall risk-benefit ratio were reviewed with the patient and his spouse, and together they agreed to a clozapine trial. After going through the necessary baseline laboratory tests and registration process, the patient was started on 12.5 mg of clozapine at bedtime. The dosage was increased by 12.5 mg/day at 2-week intervals, and at a dosage of 37.5 mg/day, the patient’s psychotic symptoms declined markedly. Several months later, the patient remained on 37.5 mg of clozapine at bedtime, and his only ongoing perceptual disturbance was the rare occurrence of fleeting visual hallucinations. The patient tolerated clozapine well, with no apparent worsening of the parkinsonian symptoms. The only side effects were tolerable mild increases in drooling and constipation.