Infections During Childhood
Although there has been a focus on risk exposures during the fetal period, there is a paucity of studies on the exposure to infections in the CNS during childhood as a potential risk factor for the future development of psychosis. This is somewhat surprising given the fact that the human brain continues to develop well into early adulthood (reviewed in reference
14 ). A Finnish follow-up of a birth cohort until 28 years of age reported no association between bacterial CNS infections during childhood and subsequent schizophrenia, whereas an increased risk was described for viral infections (odds ratio=4.8)
(15) . In a later follow-up of the same cohort up to the age of 31 years, the risk associated with viral infections was, however, attenuated (odds ratio=2.5, 95% confidence interval [CI]=0.9–7.0)
(16) . In another Finnish study, no increased risk for schizophrenia was found in a cohort consisting of 320 individuals who had suffered from viral CNS infections (the majority by enteroviruses) before their 15th birthday
(17) . In addition to the small number of exposed individuals, this study lacked a proper control group and did not include data on bacterial infections. However, in a study from Sao Paulo of 173 individuals who had suffered from “epidemic meningitis” during childhood (mainly of bacterial origin), a fivefold increase in the prevalence of psychotic disorders occurred
(18), but the study suffers from a very high dropout rate. An association between nonspecified childhood meningitis (bacterial and viral) and adult schizophrenia has also been described
(19) .
In conclusion, data concerning childhood exposures to both bacterial and viral CNS infections and the risk of subsequent psychosis are sparse and contradictory. The present study aims to investigate whether there is an increased risk of nonaffective psychotic illness associated with bacterial or viral infections in the CNS during childhood and, if so, which specific bacterial or viral agents are involved.
Materials and Methods
To address this question, we identified all children in Sweden born 1973–1985 who had contracted a CNS infection before their 12th birthday by using the Swedish National Inpatient Register, which contains data on all inpatient episodes since 1973. In addition, data on subsequent psychotic illness in the cohort until Dec. 31, 2002, was extracted. Because parental psychotic illness may be a confounder, information on parental inpatient care due to psychoses (nonaffective and/or affective) was linked from the inpatient register. By further record linkage to the medical birth register, other possible confounders were taken into account, namely urbanicity (born in a town with more than 250,000 inhabitants) and winter birth (December through March).
The following viral and bacterial CNS infections were defined as exposures with ICD-8 codes: viral infections—323 (encephalitis and encephalomyelitis), 045 (enterovirus meningitis: 04500 coxsackie, 04510 enterocytopathogenic human orphan virus, 04597 enterovirus meningitis suspecta, 04599 enterovirus not otherwise specified), 04699 (other enterovirus infections in the CNS), 05200 (varicellae meningoencephalitis), 05404 (herpes simplex meningoencephalitis), 062 (encephalitis caused by mosquitoes), 063 (encephalitis caused by ticks), 064 (virus encephalitis caused by arthropods), 065 (virus encephalitis not otherwise specified), 07201 (parotitis meningoencephalitis), 07502 (mononucleosis meningoencephalitis), 07920 (choriomeningitis lymphocytica), and 07950 (congenital cytomegalovirus, diagnosed after 1 month of age); bacterial infections—320 (purulent meningitis, 320,00 hemophilus influenzae, 320,10 penumococcum, 320,80 streptococcum, 320,88 other, 320,99 not otherwise specified), 09490 (syphilitic meningoencephalitis), 01300 (tuberculosis meningitis), and 03600 (meningococcal meningitis).
ICD-9 codes were the following: viral infections—321 (meningitis caused by viruses), 322 (nonpurulent meningitis not otherwise specified), 323 (virus encephalitis and encephalomyelitis), 046 (slow virus infection in the CNS), 047 (enterovirus meningitis: 047A coxsackie virus, 047B enterocytopathogenic human orphan virus, 047W other enterovirus, 047X enterovirus not otherwise specified), 048 (other illnesses in the CNS caused by enteroviruses), 049 (other viral CNS infections), 052W (varicellae meningitis), 053A (herpes zoster meningitis), 054D (herpes simplex encephalitis), 055A (morbilli encephalitis), 056A (rubella encephalomyelitis), 062 (encephalitis caused by mosquitoes), 063 (encephalitis caused by ticks), 064 (virus encephalitis caused by arthropods, 072B (parotitis meningitis), 072C (parotitis encephalitis), 771B (congenital cytomegalovirus, diagnosed after 1 month of age); bacterial infections—013 (tuberculosis meningitis), 036 (meningococcal meningitis and encephalitis), 094C (syphilitic meningoencephalitis), and 320 (bacterial meningitis: 320A hemophilus influenzae, 320B pneumococcum, 320C streptococcum, 320D staphylococcum, 320W other bacteria, 320X not otherwise specified).
The following diagnoses were defined as nonaffective psychotic illness: inpatient care for ICD-9 diagnoses—295 (schizophrenia), 297 (delusional syndrome), and 298 excluding A and B (reactive psychoses excluding depressive and manic psychoses); ICD-10 diagnoses—F20 (schizophrenia), F21 (pertubatio schizotypica), F22 (delusional syndrome), F23 (psychotic episode), F24 (induced delusional syndrome), F25 (schizoaffective syndrome), F28 (other psychoses), and F29 (psychosis not otherwise specified). The following diagnoses were defined as schizophrenia: inpatient care for ICD 9 diagnosis 295 (schizophrenia) and ICD-10 diagnosis F20 (schizophrenia). The national inpatient register was searched from Jan. 1, 1987 (or from the subjects’ 14th birthday), until Dec. 31, 2002 (until the persons were 17–29 years old).
Relative risk estimates and 95% CIs were calculated comparing exposed with unexposed children (children who had not had any CNS infection, whether of viral or bacterial origin) by using Mantel-Haenszel estimates. Analyses were performed crude and adjusted for age, sex, urbanicity, and a family history of psychosis. SAS software version 9.1 (SAS Institute, Cary, NC) was used in all statistical analyses. The study was approved by the research ethics committee at the Karolinska Institutet.
Results
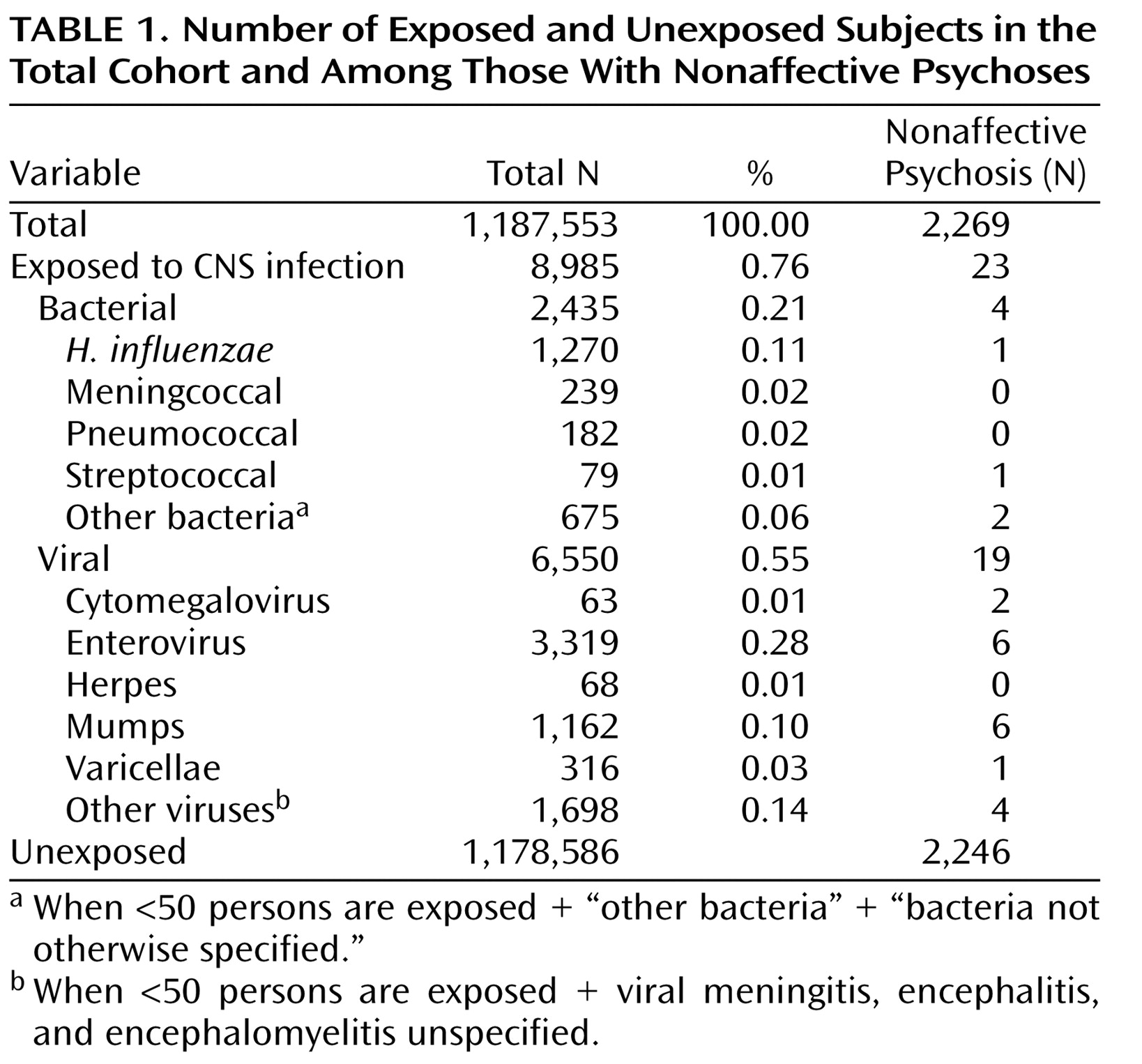
A total of 1,187,553 children were identified and followed up in the registers. Altogether, 2,435 children had been hospitalized for bacterial and 6,550 for viral CNS infections (
Table 1 ). Of the latter, 3,319 were caused by enteroviruses (50.7%) and 1,162 by the mumps virus (17.7%). Three hundred and sixteen children suffered from varicella zoster virus infections (4.8%), 63 from cytomegalovirus infections (1%) (18 children were diagnosed as having a congenital cytomegalovirus infection during their first month of life and were not included in the study), and 68 from herpes simplex virus infections (1%). Among the bacterial infections,
H. influenzae was the most common agent (52.2%). Altogether, 2,269 individuals were diagnosed with a nonaffective psychosis. Among these, 23 individuals (including eight with schizophrenia) were diagnosed with a CNS infection before the age of 12 (
Table 1 ).
Covarying Risk Factors
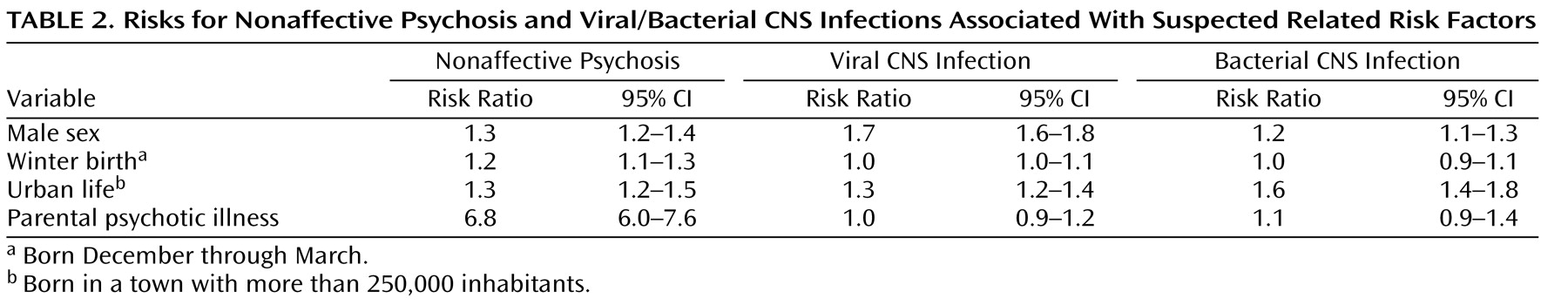
Suspected covarying risk factors are scrutinized in
Table 2, in which both the risk for psychoses
and exposure to CNS infections are presented in association with each risk factor. Male sex and urban life are associated with increased risks of both psychoses and viral/bacterial CNS infections, whereas winter birth is not. Parental psychotic illness is weakly related to bacterial CNS infection. Thus, male sex, urbanicity and parental psychotic illness covary with both the outcome and the exposure and were added into the analysis (
Table 3 ). The rationale for this is to clarify whether these covarying risk factors are explained by infection. For example, if the main risk associated with urbanicity is mediated by infection, the risk associated with infection would decrease if urbanicity were added to the analysis.
Risk for Psychoses
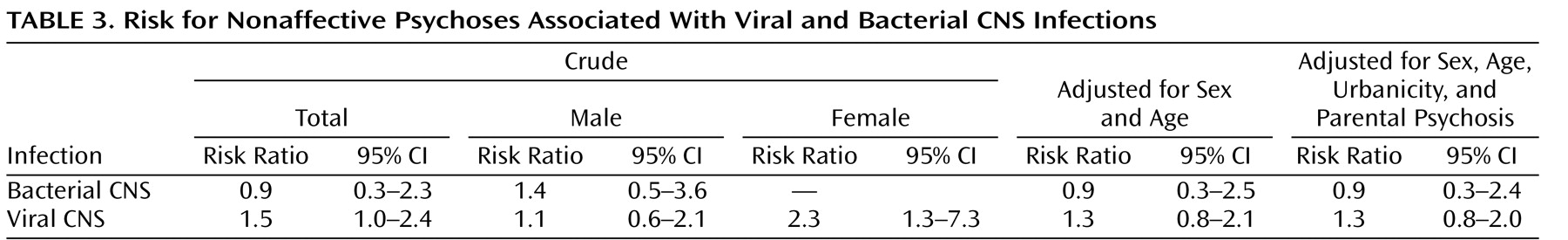
There was a slightly increased risk, at the limit of statistical significance (risk ratio=1.5, 95% CI=1.0–2.4), of developing a nonaffective psychosis (including schizophrenia) later in life if a child had been exposed to a viral CNS infection (
Table 3 ), and the risk was higher in females (risk ratio=2.3, 95% CI=1.3–7.3). Adjustment for sex and age did not affect the results substantially (risk ratio=1.3, 95% CI=0.8–2.1), nor did parental psychotic illness or urbanicity (risk ratio=1.3, 95% CI=0.8–2.0). There was no evidence of an increased risk associated with bacterial infections (risk ratio=0.9, 95% CI=0.3–2.3).
When the analyses were restricted to schizophrenia, the results were similar (data not shown). Viral CNS infection was associated with an increased risk of 1.6 (95% CI=1.0–2.5), whereas no support for an increased risk associated with bacterial infections (risk ratio=0.9, 95% CI=0.4–2.5) was found.
Specific Infectious Agents
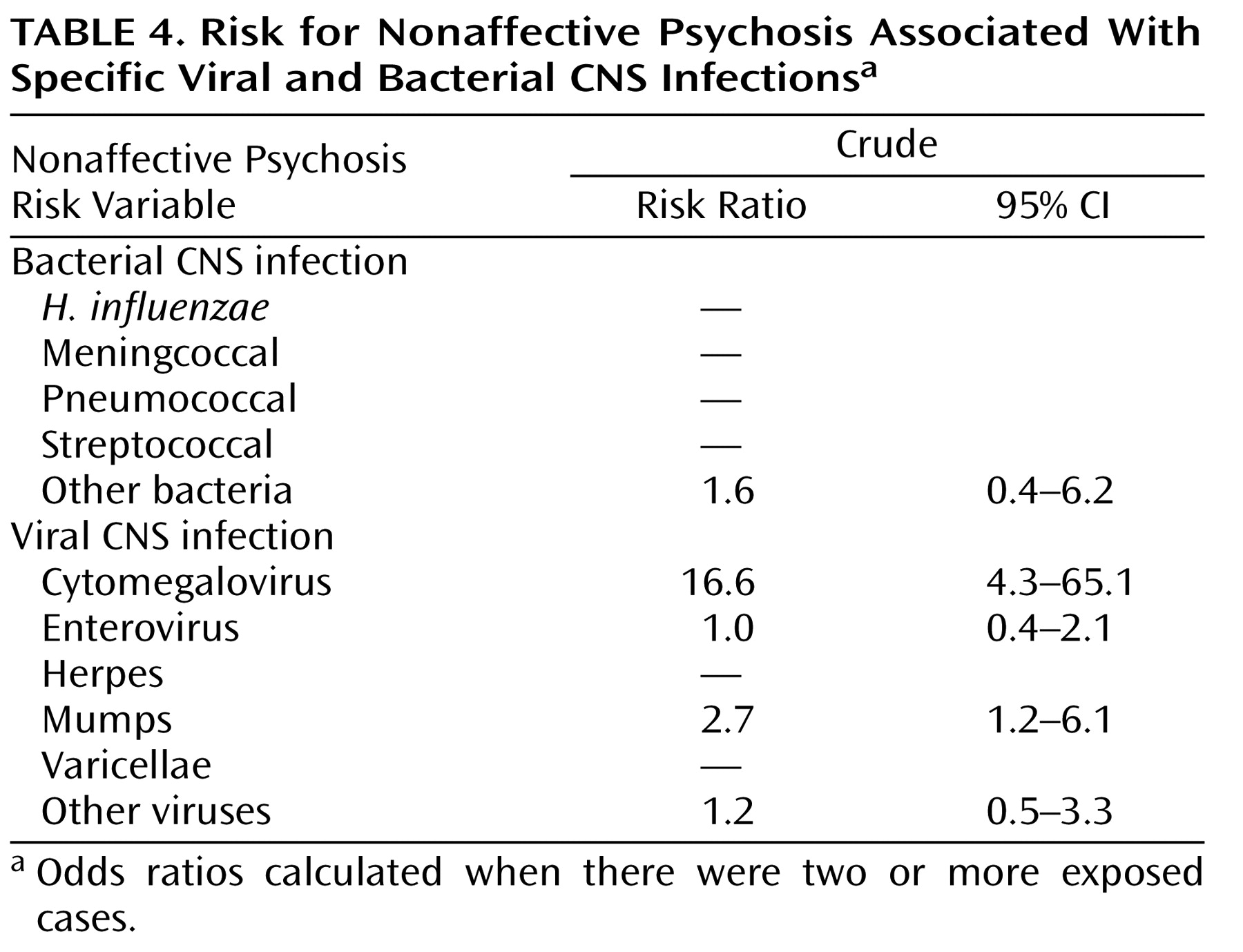
Concerning specific infectious agents (
Table 4 ), we found that the risk for nonaffective psychoses was related to CNS infections by the mumps virus (risk ratio=2.7, 95% CI=1.2–6.1) or cytomegalovirus (risk ratio=16.6, 95% CI=4.3–65.1). CNS infections caused by enteroviruses were not associated with the later development of nonaffective psychotic illness in this study (risk ratio=1.0, 95% CI=0.4–2.1).
Age of Onset and Exposure
The mean age of onset of psychoses did not differ between those exposed to viral infection and individuals not exposed (20.9 years for both groups). Among the exposed individuals, there was no difference in age at the time of infection between patients and comparison subjects (mean age=5.9 years for both groups). When categorized according to exposure to viral CNS infection before and after 6 years of age, the risk estimates did not differ significantly (1.54 and 1.49, respectively).
Description of Patients
Among the 23 individuals who were exposed to viral CNS infections during childhood and developed a psychotic illness, 14 were male (61%) and nine were female. This is in accordance with the general occurrence of CNS infections, which are more common among males (65% for viral and 57% for bacterial infections in this study).
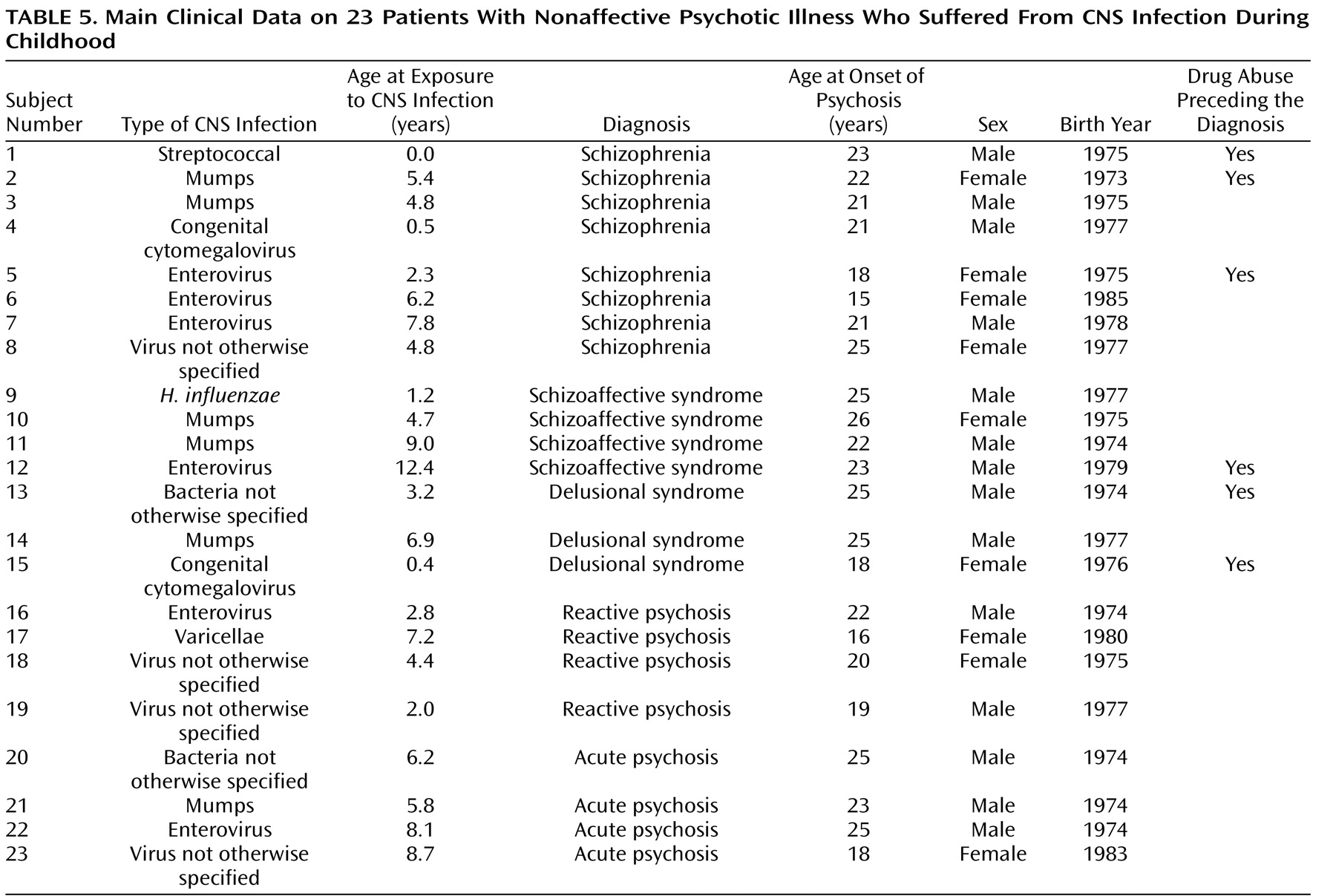
Clinical characteristics concerning exposures and outcomes are presented in
Table 5 . The age at the time of exposure was evenly distributed during childhood. The most common age at onset of psychoses was between 18 and 26 years. Eight individuals had a diagnosis of schizophrenia, and four individuals had a diagnosis of schizoaffective syndrome. Two of those with a diagnosis of schizophrenia were exposed to mumps virus and three to enterovirus. Six of the 23 patients had a history of drug abuse, most often cannabis, before being diagnosed with psychotic illness.
Discussion
In this national cohort study, we found a weak association between viral CNS infections during childhood and the later development of nonaffective psychotic illnesses. On the other hand, there was no support for an increased risk associated with bacterial CNS infections. The power to detect a doubled risk for psychosis associated with exposure to CNS infections during childhood was fairly high in this study (0.9). The power to detect increased risks of 20%–50% (odds ratio=1.2–1.5) was much smaller (0.2–0.6). Nevertheless, this is the most robust and conclusive study to date. Thus, the results suggest that the risk associated with viral CNS infections is relatively weak: less than doubled, but a null effect cannot be ruled out.
Exposure to infections was not apparently associated with any specific age of onset of psychoses nor did the age at the time of exposure seem to matter substantially. One would have expected that infections earlier in life would be more harmful than later on. However, this result is in accordance with a study from Sao Paolo
(20) .
Adding parental psychotic illness and urbanicity to the analysis had no substantial influence on the results, suggesting that these established risk factors are not mediated by infection (increased susceptibility to infections or exposures to infections). However, from
Table 2, it is evident that living in an urban area increases the risk of having CNS infections during childhood. Thus, we had expected an effect. The small numbers may be one explanation. The other is that infections are truly not related to urban risk.
Of the different viral infections, mumps and cytomegalovirus infections were associated with increased risk for psychoses in this study. It should, however, be noted that the numbers are small, and the results concerning specific infectious agents should therefore be interpreted with caution. Nevertheless, it is of interest to note that mumps before vaccination was the single most common cause of aseptic meningitis or mild encephalitis, 15% of all cases
(21) . This figure is similar to the 17% observed in the present material from Sweden, where general vaccination against measles, mumps, and rubella viruses was first introduced in 1982
(22) .
Mumps virus can be genotyped according to sequence variations in the gene encoding the small hydrophobic protein of the virus, and currently, 12 different genotypes, designated A–L, with varying degrees of neurovirulence have been identified
(23) . During the years 1973–1985, strains of genotypes C and D were circulating in Sweden, and these appear to be more neurovirulent than those of genotype A
(24) . In a study of 18 patients infected with mumps strains of genotypes C or D, Tecle and coworkers
(24) reported that nine patients suffered from meningitis only and nine had a combination of parotitis and meningitis, whereas none suffered from parotitis only. This contrasts markedly with infections with the genotype A, which caused parotitis only in the vast majority of the patients
(24) . From earlier reports, it is also well known that mumps virus can be highly neurovirulent. During certain epidemics, more than 50% of patients with parotitis showed pleocytosis in the CSF, whereas approximately half of the number of patients with mumps, meningitis, or encephalitis may show no signs of parotitis (reviewed in reference
3 ). Thus, different risk estimates for mumps virus can potentially be obtained for different circulating strains.
Mumps virus probably invades the brain across the choroid plexus, from which the virus spreads through the ventricles to cause infection of ependymal cells (reviewed in reference
25 ). In experimental models, neurovirulent genotypes of mumps virus may cause hydrocephalus
(20) . In such models, neurovirulent genotypes can also cause persistent infections of neurons and induce developmental disturbances (for a review, see reference
26 ). According to the report by Julkunen and coworkers
(27), the frequency or severity of sequelae following mumps encephalitis can be high. In their Finnish cohort, 23 of 47 patients examined 1–15 years after the disease experienced difficulties in memory and learning, focal motor or sensory signs, or loss of hearing and visual acuity. Chronic mumps virus CNS infections in humans have also occasionally been described
(28,
29) . In addition to these effects of mumps on the nervous system, we suggest that mumps infections during childhood can be associated with an increased risk for the future development of nonaffective psychosis. Although mumps virus antigens have never been found in brains from patients with schizophrenia postmortem, abnormal antibody titers to the virus in CSF have been reported (reviewed in reference
30 ).
Cytomegalovirus infection was also found to confer an increased risk for the future development of nonaffective psychotic illness (risk ratio=16.6, 95% CI=4.3–65.1), although only two patients were identified in the present material. It should be noted that of the 81 individuals having a diagnosis of congenital cytomegalovirus infection, only 18 were diagnosed during their first month of life. Since these children were presumed to be infected prenatally, they were excluded from the current study. In a prospective study conducted in Malmö, Sweden, during the late 1970s and early 1980s, 35% of the children who tested negative at birth shed virus in the urine already at 6 months of age
(31), indicating a high rate of infection during infancy. Consequently, the majority of the 63 children diagnosed at older ages were probably infected postnatally. The two children who developed nonaffective psychosis in the present material were diagnosed at 5 and 6 months of age, respectively. With regard to the long-term outcome of cytomegalovirus infections, the age at the time of infection is a major determinant. Although congenital cytomegalovirus infections are considered the leading infectious cause of brain damage in children
(32), postnatally acquired cytomegalovirus infection is believed to be far more harmless
(33,
34) . However, studies on the long-term consequences of postnatally acquired cytomegalovirus infections are missing
(34) . The present study suggests that delayed psychiatric symptoms should be considered in future studies. Although cytomegalovirus genomes have not been found in postmortem brains from schizophrenic patients
(35,
36), an association between recent onset and deficit schizophrenia and cytomegalovirus antibody seropositivity has been described
(37,
38) . Like mumps virus, cytomegalovirus is thought to infect cells in periventricular regions followed by invasion of the brain parenchyma (reviewed in reference
39 ).
Methodological Concerns
The strength of the current study is the longitudinal design with data collected prospectively, which minimize the risk for bias. On the other hand, a potential weakness of the study is that the diagnoses are register based, although in a validation study, the register diagnoses of schizophrenia proved to be in accordance to DSM-IV in 82% of the cases
(40) . Furthermore, the Swedish National Discharge Register was not complete during the first years of the study; therefore, data from seven of 26 county councils are missing for 1973, but we have no reason to believe that this lack of data is selective. In this study, exposure is defined as inpatient care for CNS infections. It is, therefore, likely that many cases of mild meningitis/encephalitis pass without detection. On the other hand, the different diagnoses of CNS infections appear to be accurate in the Swedish National Discharge Register
(41) . Consequently, this study describes only the risk associated with register-based CNS infections requiring hospital treatment. The same applies to the patients with a diagnosis of psychiatric disease, which encompass only hospital-treated cases and therefore most likely include patients with more severe symptoms than those observed in outpatients, which were not included in the present study.
Finally, although the study population consisted of 1.2 million children, our power was limited to detect increased risks of a risk ratio less than 1.5. Nevertheless, very strong associations may be ruled out.
In conclusion, this study shows that serious viral CNS infections during childhood may be associated with the later development of schizophrenia and nonaffective psychosis. Our finding that an elevated risk is associated with mumps virus and cytomegalovirus infections indicates that the risk relates to infectious agents with a propensity to invade the brain parenchyma rather than to CNS infections in general.