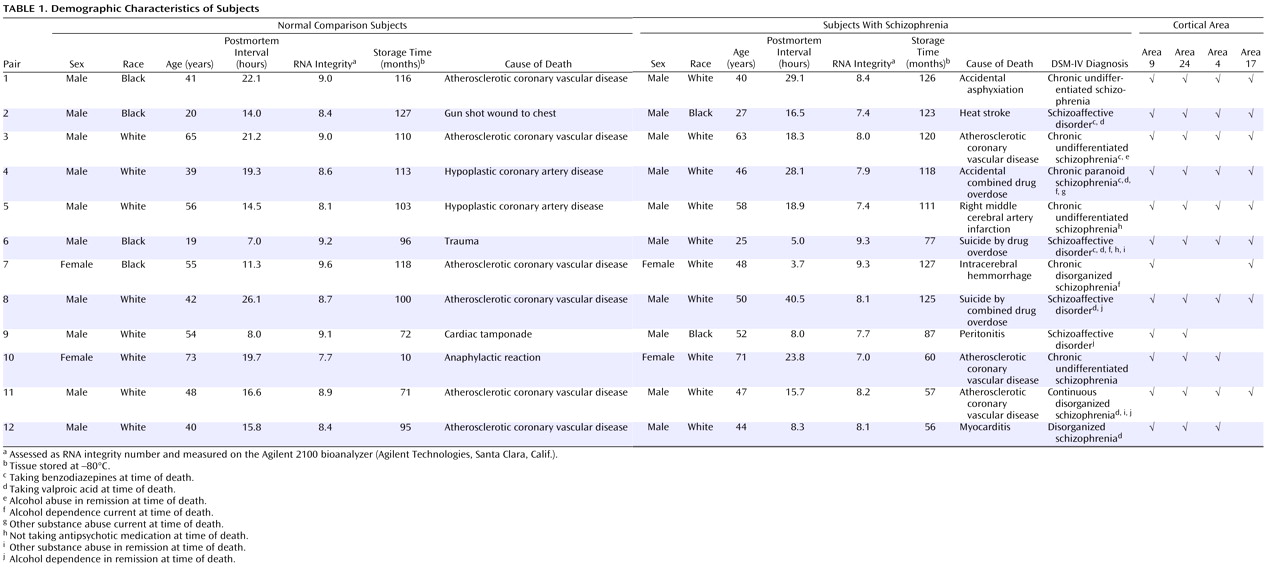
The core features of schizophrenia include disturbances in critical cognitive functions, such as working memory, that are mediated by the neural circuitry of the dorsolateral prefrontal cortex
(1,
2) . In the dorsolateral prefrontal cortex of subjects with schizophrenia, markers of inhibitory neurotransmission appear to be impaired
(3) . For example, reduced levels of mRNA encoding the 67-kilodalton isoform of glutamic acid decarboxylase (GAD
67 ), the enzyme principally responsible for GABA synthesis
(4), and the GABA membrane transporter GAT-1, which regulates the reuptake of synaptically released GABA, have been replicated in multiple postmortem studies of schizophrenia
(5 –
12) . These alterations in markers of GABA neurotransmission appear to involve specific subsets of GABA neurons. For example, mRNA encoding parvalbumin and somatostatin, each of which is expressed in a separate subset of GABA neurons, was decreased, whereas mRNA encoding calretinin, which is expressed in a third subset of GABA neurons, was unchanged in subjects with schizophrenia
(11,
13) . Furthermore, reduced GABA synthesis might be selectively mediated by a deficit in GAD
67, because neither mRNA nor protein levels of GAD
65, which contributes to GABA synthesis only under conditions of high synaptic demand
(4), were altered in the dorsolateral prefrontal cortex of subjects with schizophrenia
(8) . In addition to these presynaptic markers of GABA neurotransmission, we recently reported decreased mRNA expression for several GABA
A receptor subunits in the dorsolateral prefrontal cortex of subjects with schizophrenia
(11), including the α1 and δ subunits, the major subunits in synaptic and extrasynaptic cortical GABA
A receptors, respectively. Because normal working memory function depends upon GABA-mediated neurotransmission in the dorsolateral prefrontal cortex
(14), these molecular alterations are thought to contribute to working memory disturbances in subjects with schizophrenia
(3) .
Individuals with schizophrenia also exhibit abnormalities in other cognitive, affective, sensory, and motor functions that depend on the circuitry of other cortical regions
(15 –
17) . Therefore, we sought to determine whether the specific pattern of changes in GABA-related transcript expression observed in the dorsolateral prefrontal cortex (i.e., decreased mRNA expression for GAD
67, GAT-1, parvalbumin, somatostatin, GABA
A α1, and GABA
A δ and unaltered mRNA expression for calretinin and GAD
65 ) is specific to this area only or could also contribute to the dysfunction of other cortical areas in subjects with the illness. Consequently, we assessed the levels of these eight transcripts in four cortical areas (the dorsolateral prefrontal cortex, the anterior cingulate cortex, and the primary motor and primary visual cortices) of subjects with schizophrenia and matched normal comparison subjects. Because these areas represent the major functional domains of the cerebral cortex (e.g., association, limbic, motor, and sensory functions, respectively), they provide a robust test of the hypothesis that a conserved set of molecular alterations underlie the pathophysiology of different clinical features of schizophrenia.
Discussion
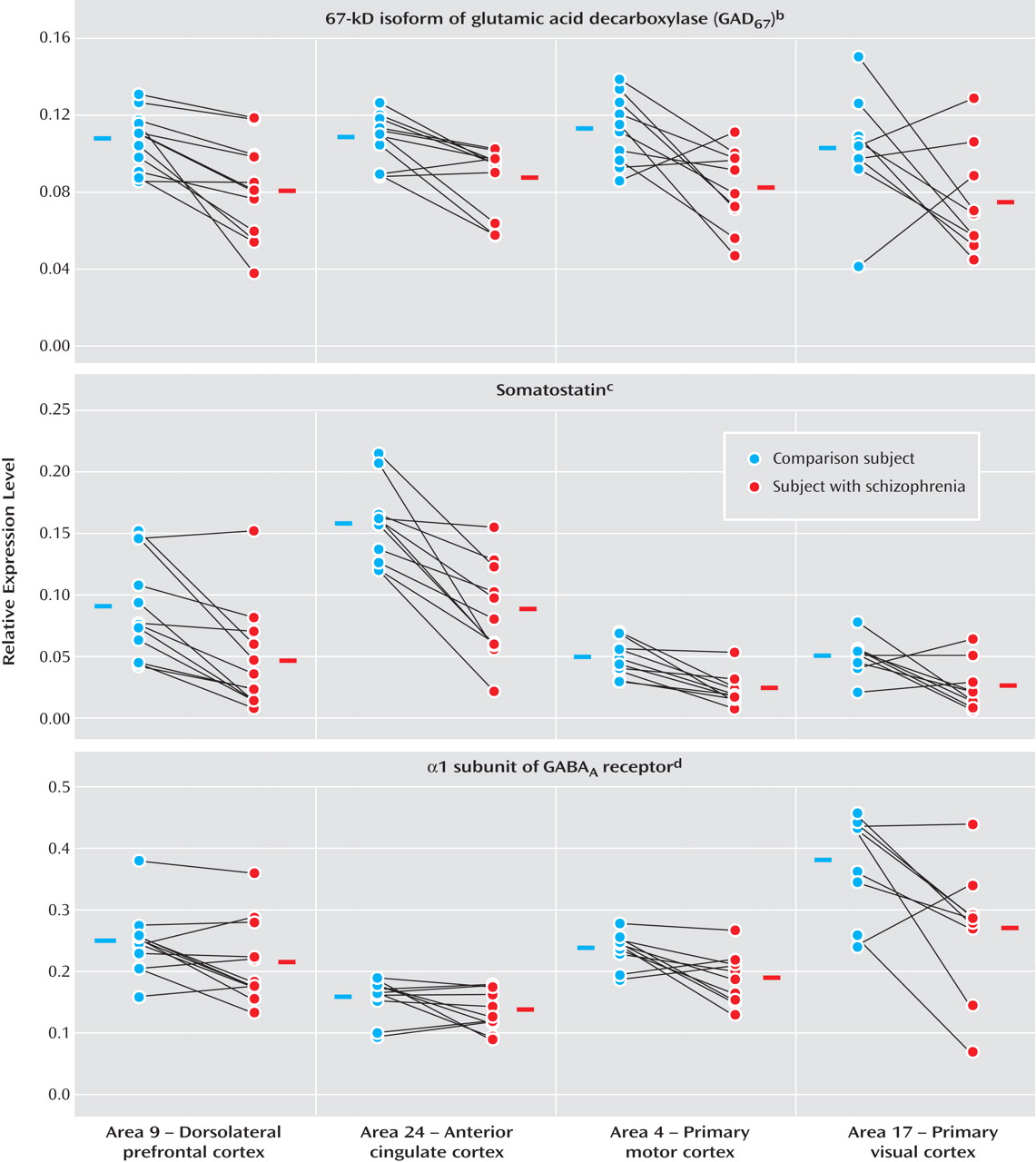
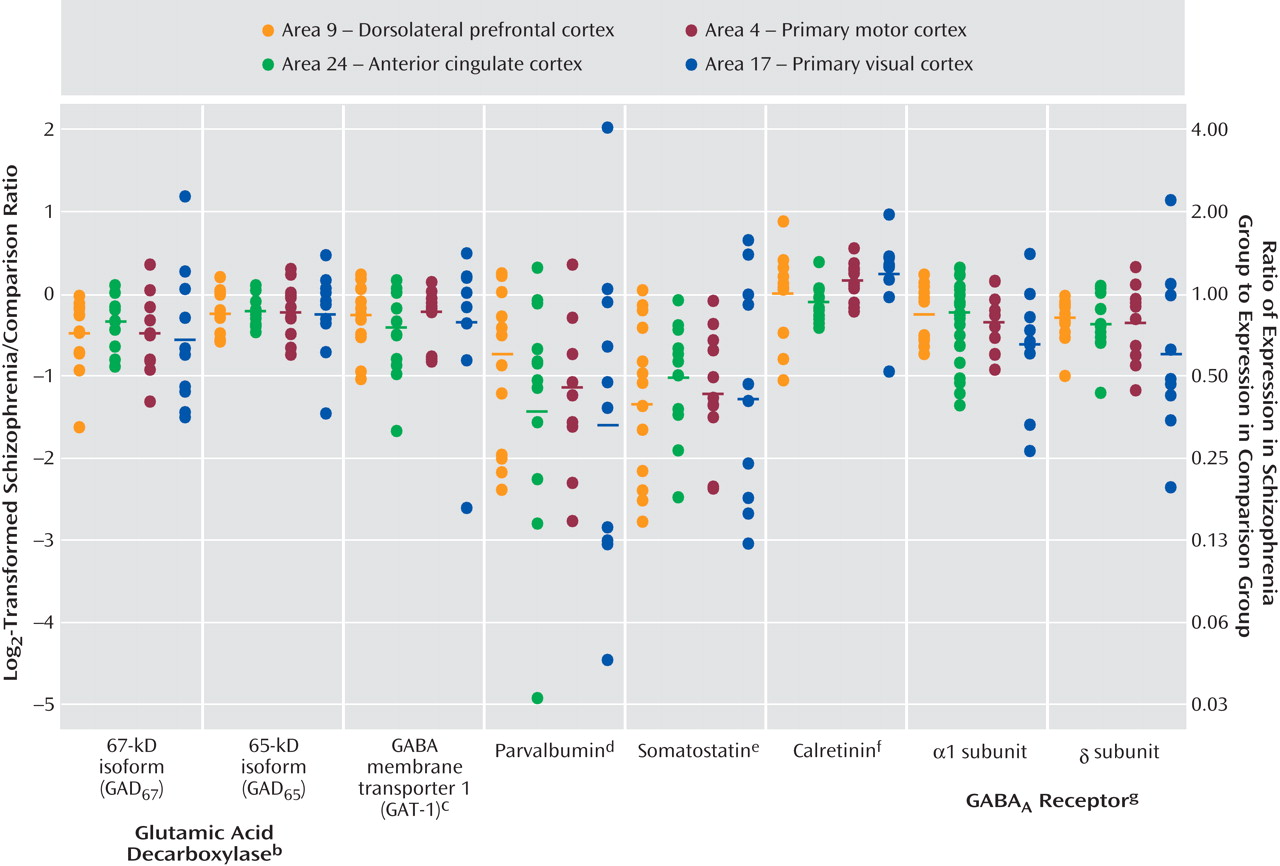
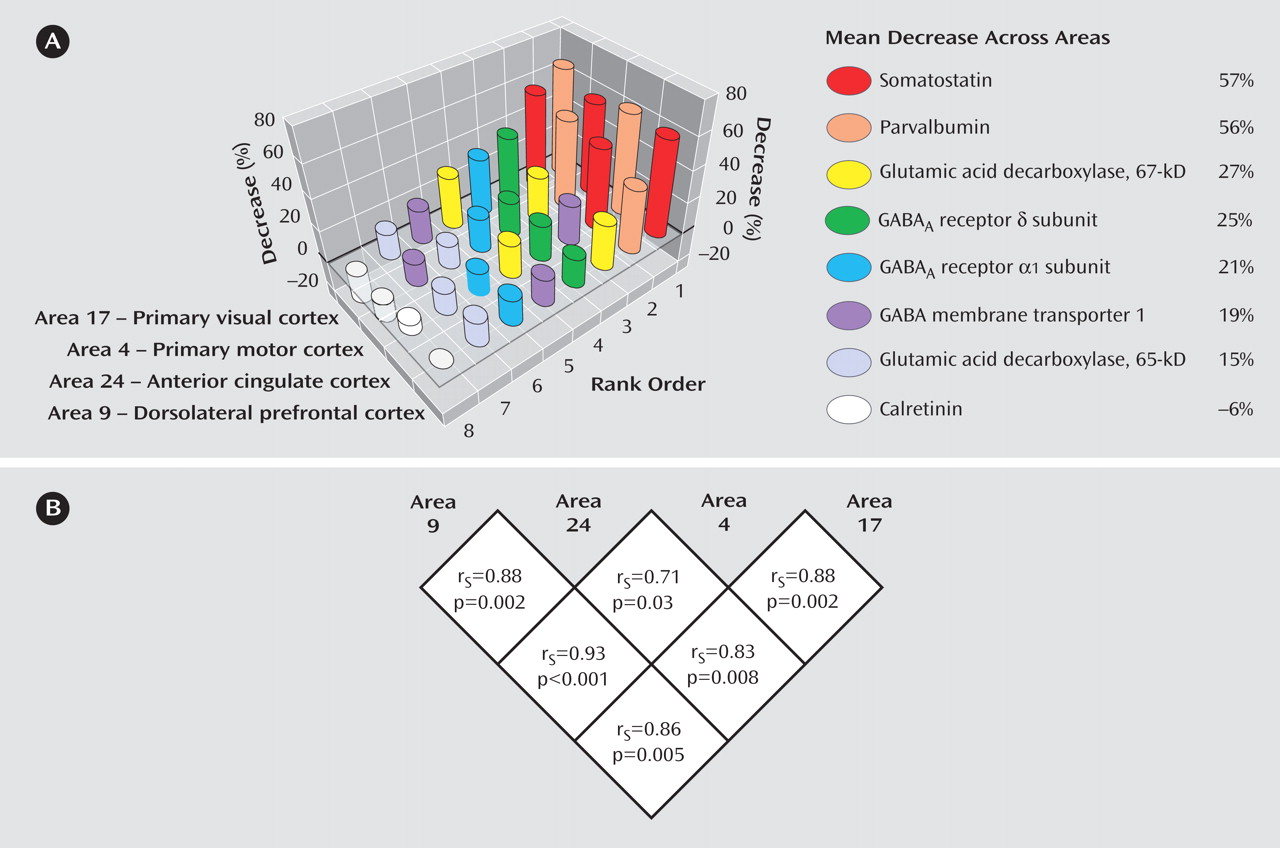
Expression levels of seven GABA-related transcripts were reduced in subjects with schizophrenia in each of four cortical regions, with the magnitude of decrease for each transcript comparable across the four cortical areas. The largest decreases were detected for mRNA encoding somatostatin and parvalbumin, each of which is selectively expressed by a distinct subset of GABA neurons. General mediators of GABA neurotransmission, such as GAD
67 and GAT-1 mRNA (which are expressed by all GABA neurons) and GABA
A receptors α1 and δ subunits (which are expressed by both GABA and pyramidal neurons), showed moderate decreases in subjects with schizophrenia. mRNA for GAD
65 exhibited the smallest, although still significant, decrease across the four areas (
Figure 3 ). In contrast, the expression of calretinin mRNA, which is expressed in ∼50% of GABA neurons that contain neither parvalbumin nor somatostatin
(21), did not differ between subject groups in any of the four areas.
These findings suggest that intrinsic cortical GABA neurotransmission in schizophrenia, with selective involvement of the parvalbumin- and somatostatin-containing subsets of GABA neurons, is altered in a similar manner across cortical regions that are markedly different in cytoarchitecture, connectivity, and function. Furthermore, impaired GABA neurotransmission is unlikely to be restricted to the cortical areas or subjects evaluated in this study, since reductions in GAD
67 and GAT-1 mRNA and/or protein have been previously reported in the auditory cortices of the superior temporal gyrus and in the anterior cingulate cortex in other subject cohorts
(22 –
24) . Therefore, impaired cortical GABA neurotransmission in schizophrenia is not likely due to factors more characteristic of altered dorsolateral prefrontal cortex (relative to at least some other cortical regions) circuitry in schizophrenia, such as reduced pyramidal cell dendritic spine density
(18) or reduced excitatory input from the hippocampus
(25) or mediodorsal thalamus
(26) . Instead, the conserved pattern of alteration in transcript levels is consistent with the presence of one or more common upstream mechanisms that are operative across cortical areas. For example, polymorphisms in genes encoding GAD
67 (12) and GABA
A receptor subunits
(27) have been associated with both schizophrenia and alterations in their transcript levels. Furthermore, a recent study indicated that the signaling of neuregulin-1 through its receptor ErbB4, both of which are the products of putative susceptibility genes in schizophrenia
(28), regulates inhibitory neurotransmission by a subset of cortical GABA neurons
(29) . Finally, parvalbumin- and somatostatin-containing cortical GABA neurons originate within the medial ganglionic eminence, whereas calretinin-containing GABA neurons are derived from the caudal ganglionic eminence
(30) . In addition, both time of birth
(30) and transcription factors that regulate early differentiation
(31) differ across these subpopulations of GABA neurons. Therefore, alterations in factors that are associated with the origin or maturation of both parvalbumin- and somatostatin-containing, but not calretinin-containing, GABA neurons could account for both cell type specificity and regional conservation of GABA neuron disturbances in schizophrenia.
The results of this study also address the question of whether cortical pathology is more pronounced in the dorsolateral prefrontal cortex than in other cortical regions in subjects with schizophrenia. For example, previous studies found that both the size of neurons
(32) and the density of pyramidal neuron dendritic spines
(18) were significantly decreased in the dorsolateral prefrontal cortex, but not the primary visual cortex, of subjects with schizophrenia. However, our current observations do not support the idea of preferential involvement of the dorsolateral prefrontal cortex, at least with regard to GABA-related transcript expression. Interestingly, we have previously found conserved reduction in the somal volume of layer 3 pyramidal neurons across the dorsolateral prefrontal cortex in Brodmann’s area 9, primary auditory area 41, and auditory association area 42 in subjects with schizophrenia
(33 –
35) . Thus, at least several types of cortical pathology, affecting both inhibitory and excitatory neurons, might commonly be present across different cortical areas regardless of their functional properties, whereas other types of pathology might be relatively region-specific.
Evidence indicates that the reduced expression of GABA-related transcripts in schizophrenia is not attributable to the pharmacological treatment of the illness. First, transcript levels for GAD
67, somatostatin, and GABA
A α1 and δ subunits were not altered in the dorsolateral prefrontal cortex of monkeys chronically exposed to typical (haloperidol) or atypical (olanzapine) antipsychotic medications that produce trough plasma levels in the therapeutic range for humans
(11) . Second, transcript levels for GAD
67, GAT-1, parvalbumin, and somatostatin were not altered in the dorsolateral prefrontal cortex of a different set of monkeys exposed to haloperidol at higher plasma levels, which produced marked extrapyramidal symptoms and required treatment with benztropine mesylate
(7,
9,
13) . Third, in our previous studies, transcript levels for GAD
67, GAT-1, parvalbumin, somatostatin, and GABA
A α1 and δ subunits were reduced in the dorsolateral prefrontal cortex of subjects with schizophrenia who were not taking antipsychotic medication at the time of death
(11) . Finally, in the present study, each GABA-related transcript showed comparable magnitudes of decrease across the four cortical areas, all of which differ both in the density of dopamine afferents that they receive
(36) and in their expression of dopamine receptors
(37) and are thus likely to be differentially influenced by antipsychotics.
The available data also indicate that the observed transcript changes are not driven by other confounding factors commonly associated with the illness. For example, in the present study (data supplement Figure 2) and in our previous microarray studies
(11), we did not observe a significant effect of alcohol abuse/dependence or the use of benzodiazepines/mood stabilizers on decreases in GAD
67, GAD
65, GAT-1, parvalbumin, somatostatin, GABA
A a1, and GABA
A d mRNA levels in any cortical area. Thus, it is unlikely that exposure to substances whose psychotropic effects are mediated through GABA neurotransmission produced the observed transcript changes. In addition, our previous studies clearly demonstrated that decreases in GAD
67, GAT-1, parvalbumin, somatostatin, GABA
A a1, and GABA
A d mRNA levels were not influenced by death by suicide in subjects with schizophrenia
(11) .
The impact of impaired GABA neurotransmission, especially via parvalbumin- and somatostatin-containing GABA neurons, on information processing in the dorsolateral prefrontal cortex may reveal how the conserved regional pattern of GABA-related transcript alteration observed in the present study could contribute to cortical dysfunction more broadly in schizophrenia. The network of parvalbumin-containing neurons, formed via both chemical and electrical synapses, plays a central role in generating oscillatory activities at the gamma band range (30–80 Hz)
(38), whereas the network of somatostatin-containing neurons appears to be important for producing oscillations at the theta range (4–7 Hz)
(39) . In the dorsolateral prefrontal cortex, gamma band oscillations are induced and sustained during the delay period in working memory tasks
(40), and their power increases in proportion with memory load
(41), indicating that gamma band oscillations are associated with local neuronal processing critical for the maintenance and manipulation of information. On the other hand, theta band oscillations are recorded across widely distributed areas during working memory tasks
(42), indicating their importance in orchestrating global interactions among distributed neuron networks. Together, these findings suggest that altered GABA neurotransmission by parvalbumin- and somatostatin-containing GABA neurons would have marked effects on the oscillatory activity of cortical neuronal networks required for dorsolateral prefrontal cortex functions. Consistent with this interpretation, recent studies have demonstrated impaired performance and reduced frontal gamma
(43) and theta
(44) activity in subjects with schizophrenia during cognitive tasks. Interestingly, cortical oscillations, especially at the gamma band range, have also been associated with primary motor
(45) and visual
(46) processing, and abnormal visual perception accompanied by reduced gamma band activity has been reported in occipital cortical areas of individuals with schizophrenia
(47) . Furthermore, abnormal gamma and theta oscillations observed in schizophrenia subjects during auditory processing tasks
(48) might reflect altered GABA neurotransmission in auditory cortices
(23,
24) . Therefore, altered GABA neurotransmission mediated by parvalbumin- and somatostatin-containing GABA neurons may contribute to the dysfunction of multiple cortical areas in subjects with schizophrenia by disturbing cortical oscillations.
In addition to altered transcript expression in GABA neurons, this study revealed comparable reductions across cortical areas in mRNA levels for GABA
A receptor α1 and δ subunits, both of which are expressed in cortical pyramidal and GABA neurons
(49) . Because α1 and δ subunits are frequently present in synaptic and extrasynaptic receptors, respectively
(50), these findings suggest that reduced phasic (synaptic) as well as tonic (extrasynaptic) inhibition in both pyramidal and GABA neurons might be a feature shared by multiple cortical areas. Although it remains to be determined if these changes represent a primary pathology affecting postsynaptic GABA signaling or a process secondary to the alteration of presynaptic GABA neurons, reduced phasic and tonic inhibition appear to influence the selection and integration of excitatory inputs to cortical neurons
(50) and could contribute to impaired information processing in each cortical area.
In summary, the findings of this study suggest that disturbances in the expression of a set of GABA-related mRNA commonly found in the dorsolateral prefrontal cortex in subjects with schizophrenia are conserved across at least three other regions of the neocortical mantle. Thus, disturbances in GABA neurotransmission by specific cell types through certain types of GABA A receptors could represent a common pathophysiology for different domains of cortical dysfunction in schizophrenia, raising the possibility that pharmacological agents with the appropriate specificity for certain GABA-related targets might be effective for a range of clinical features of the illness.